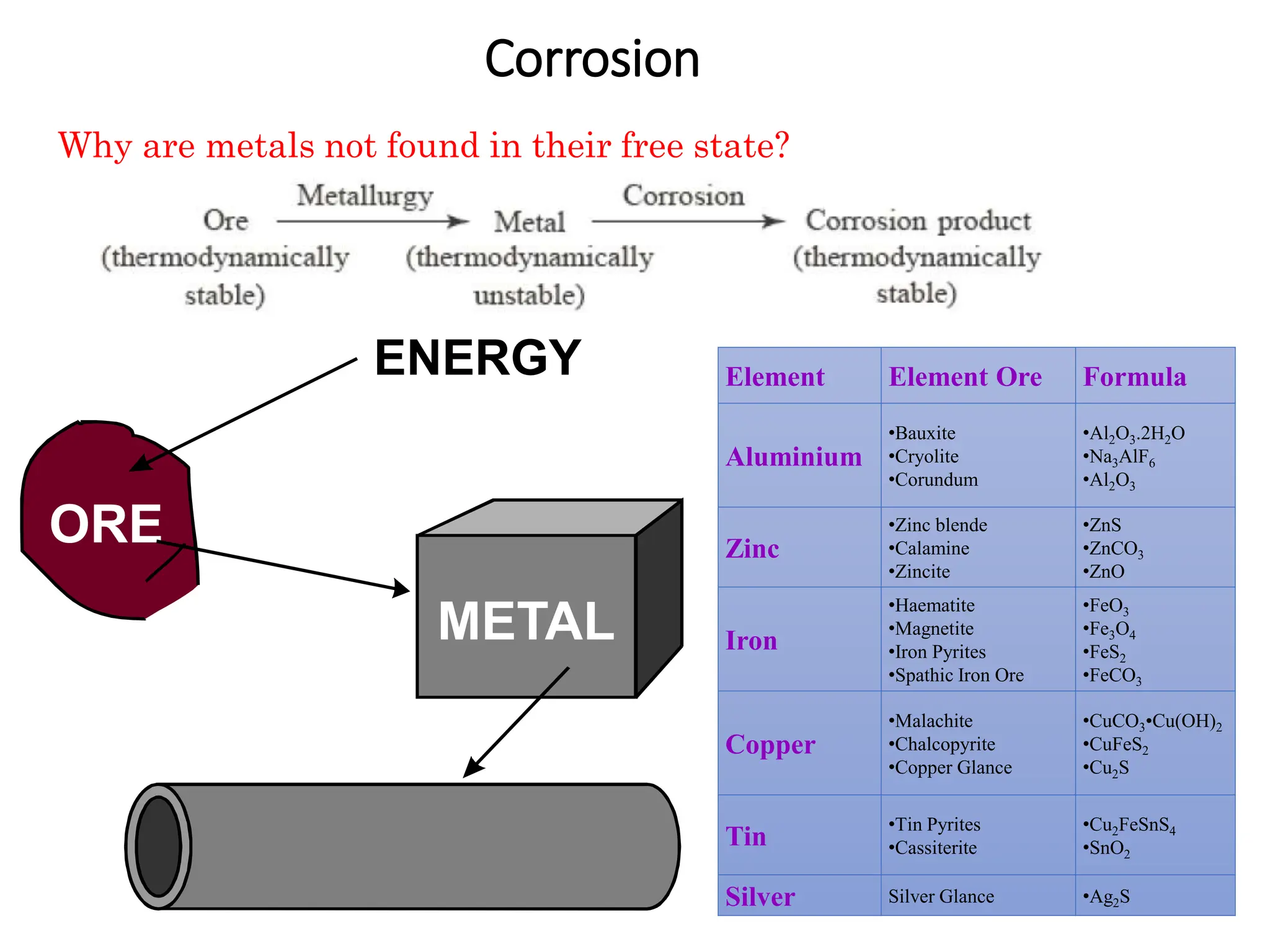
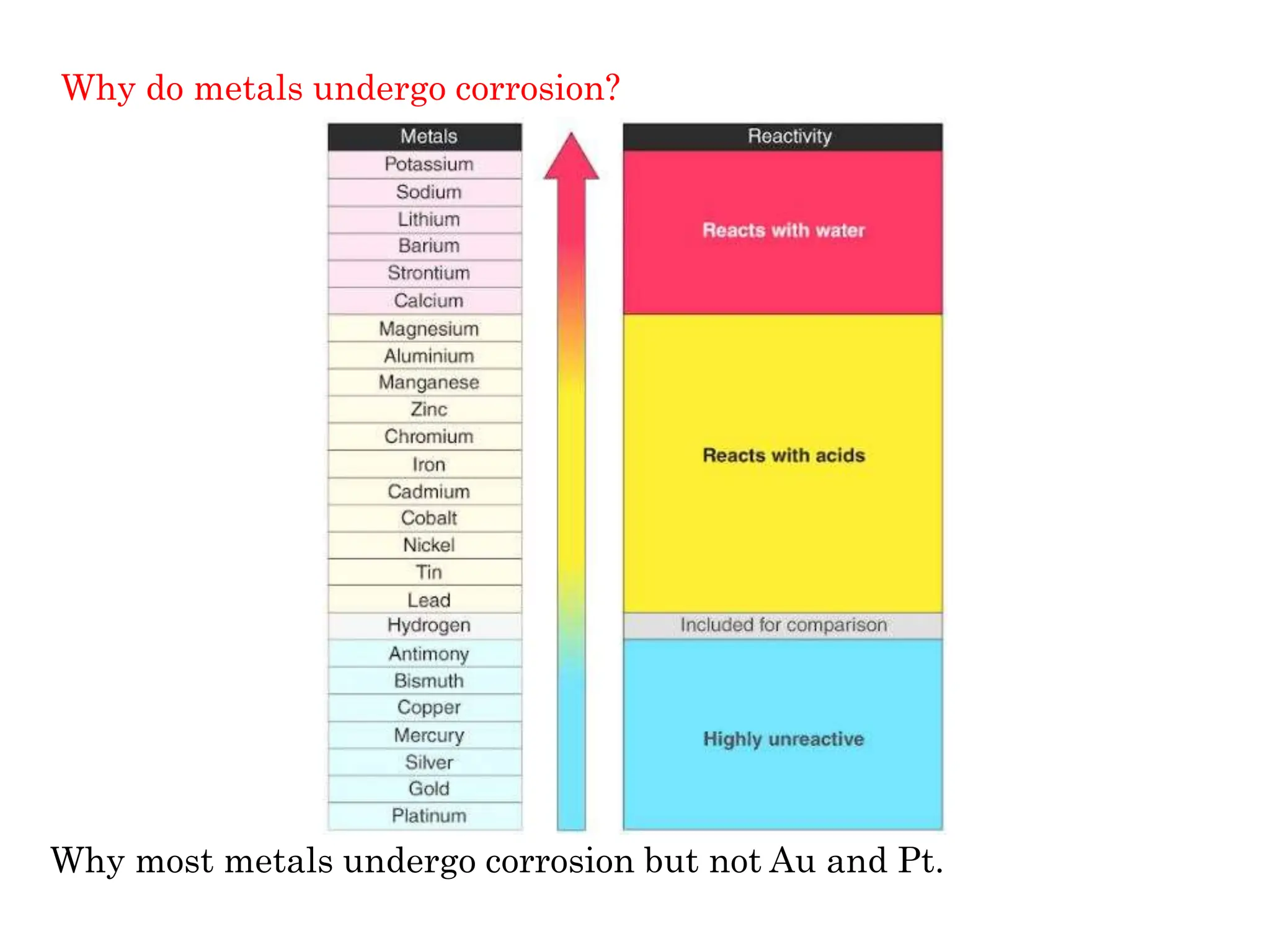
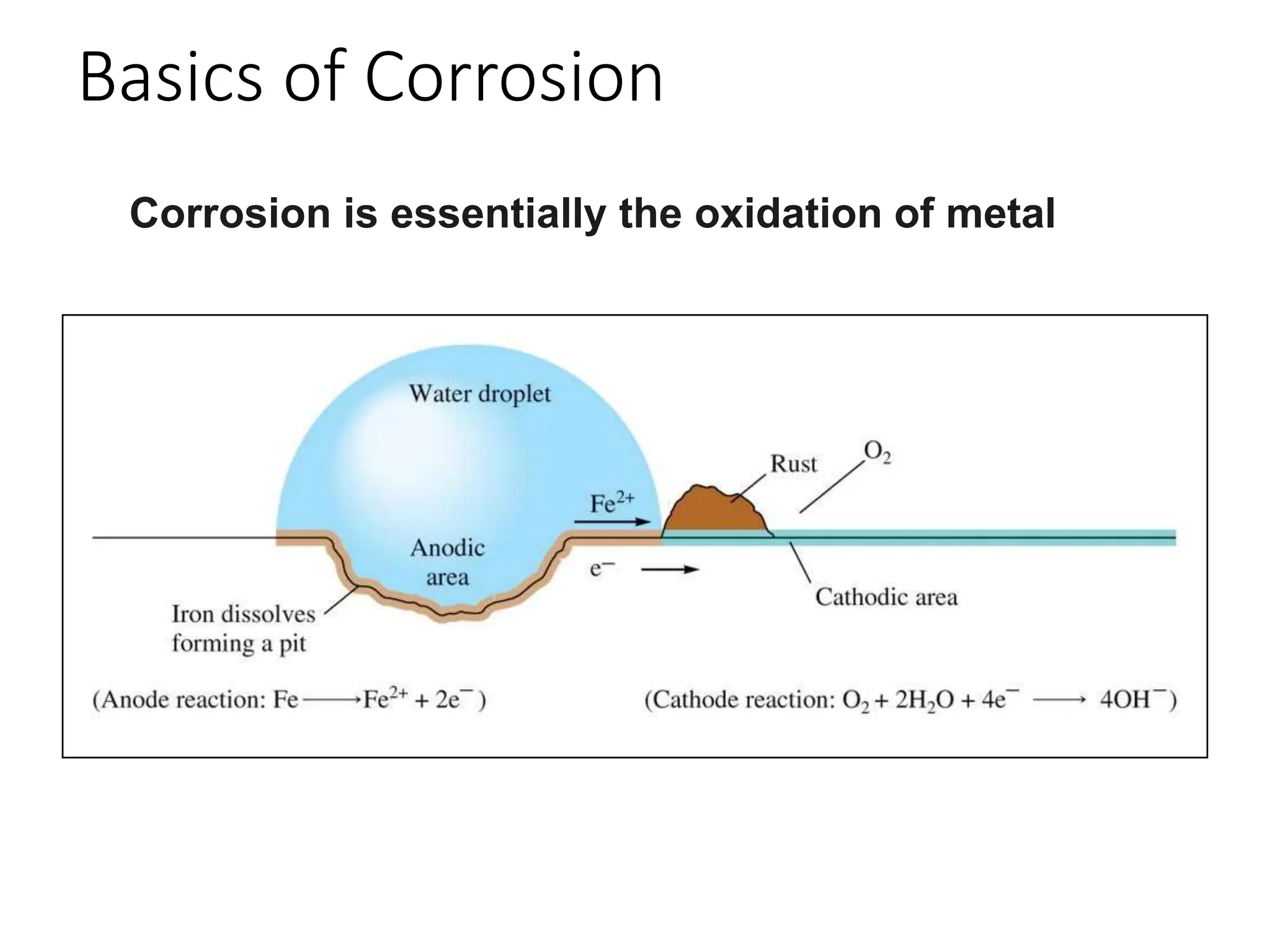
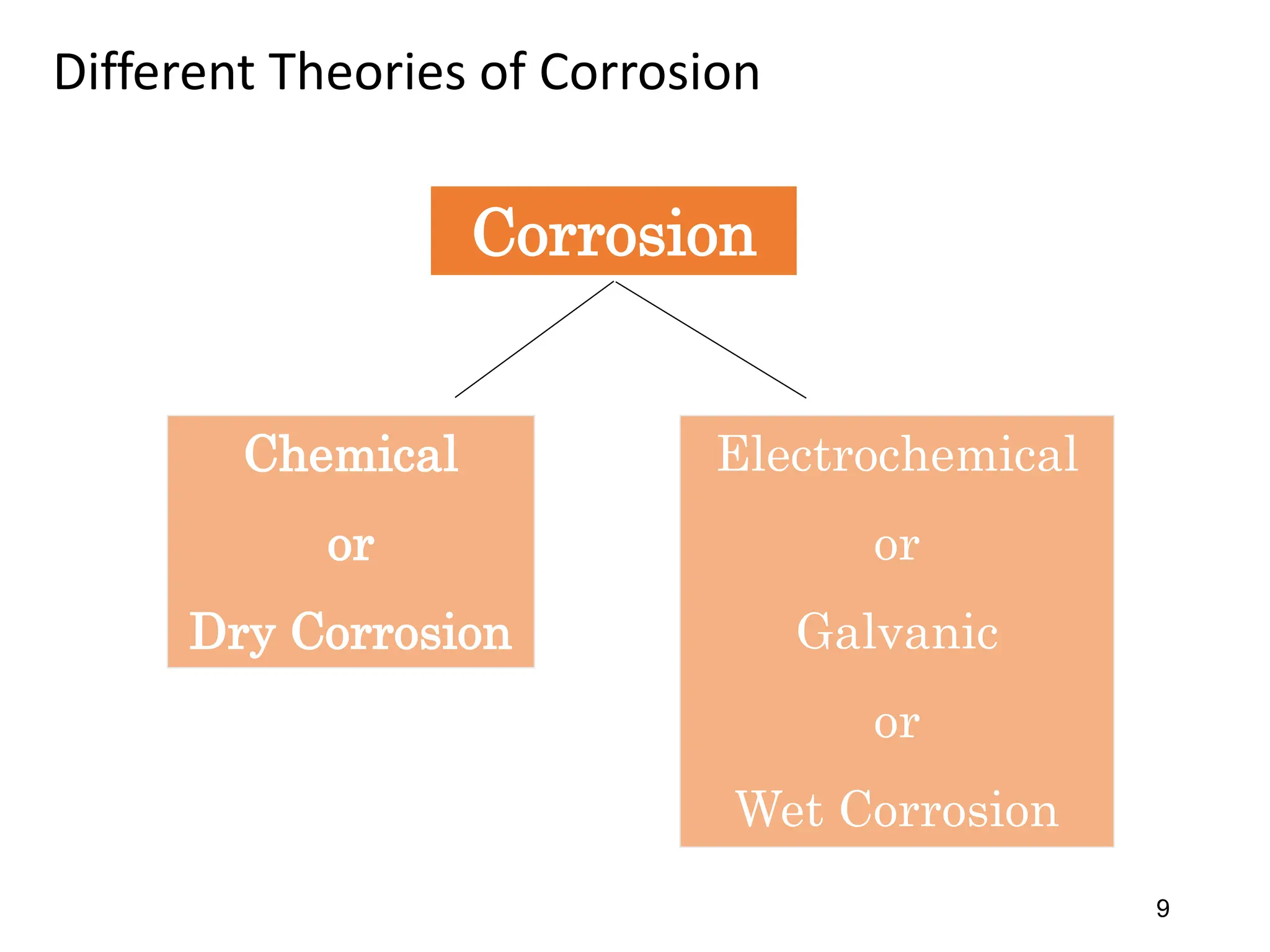
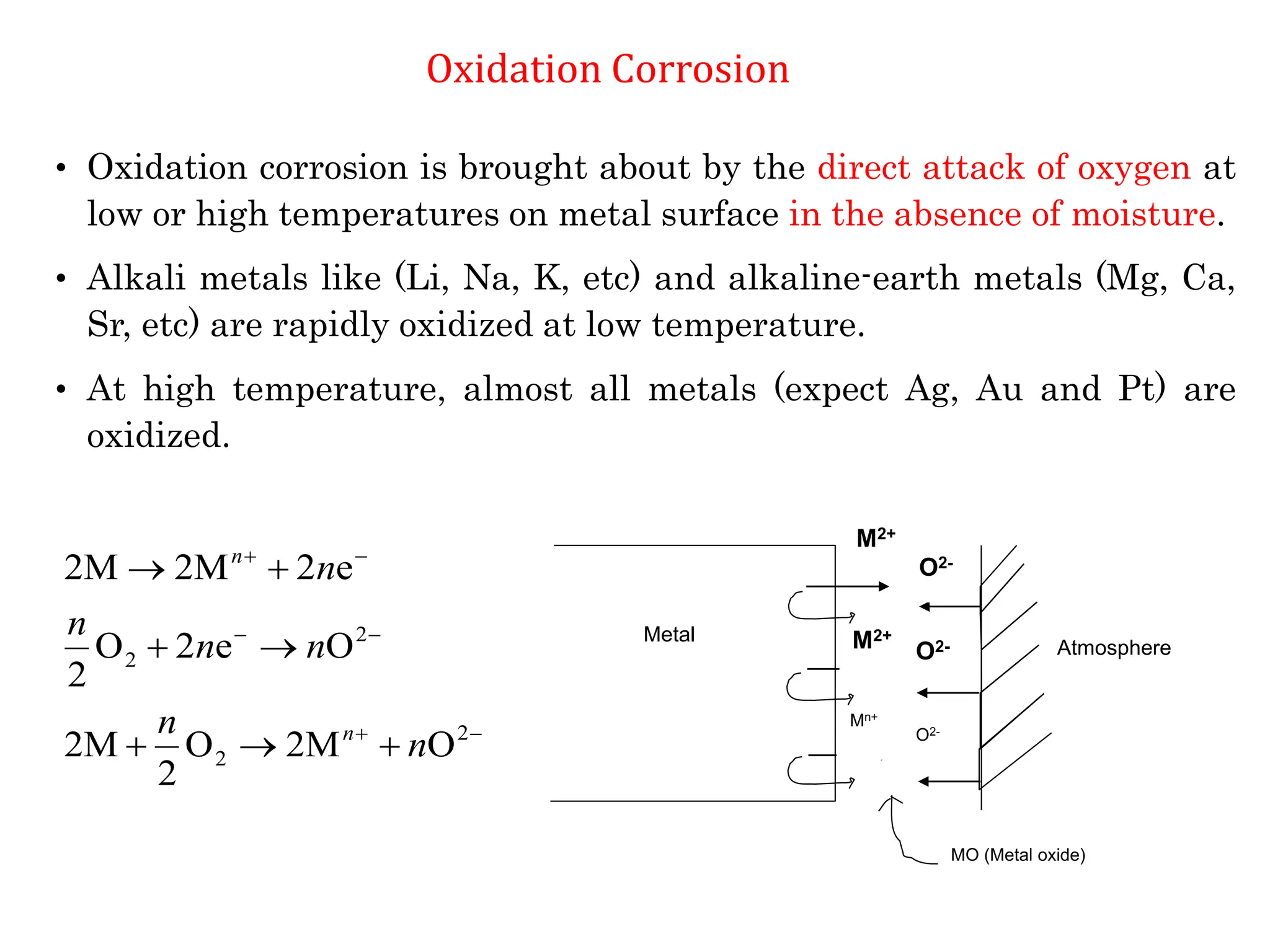
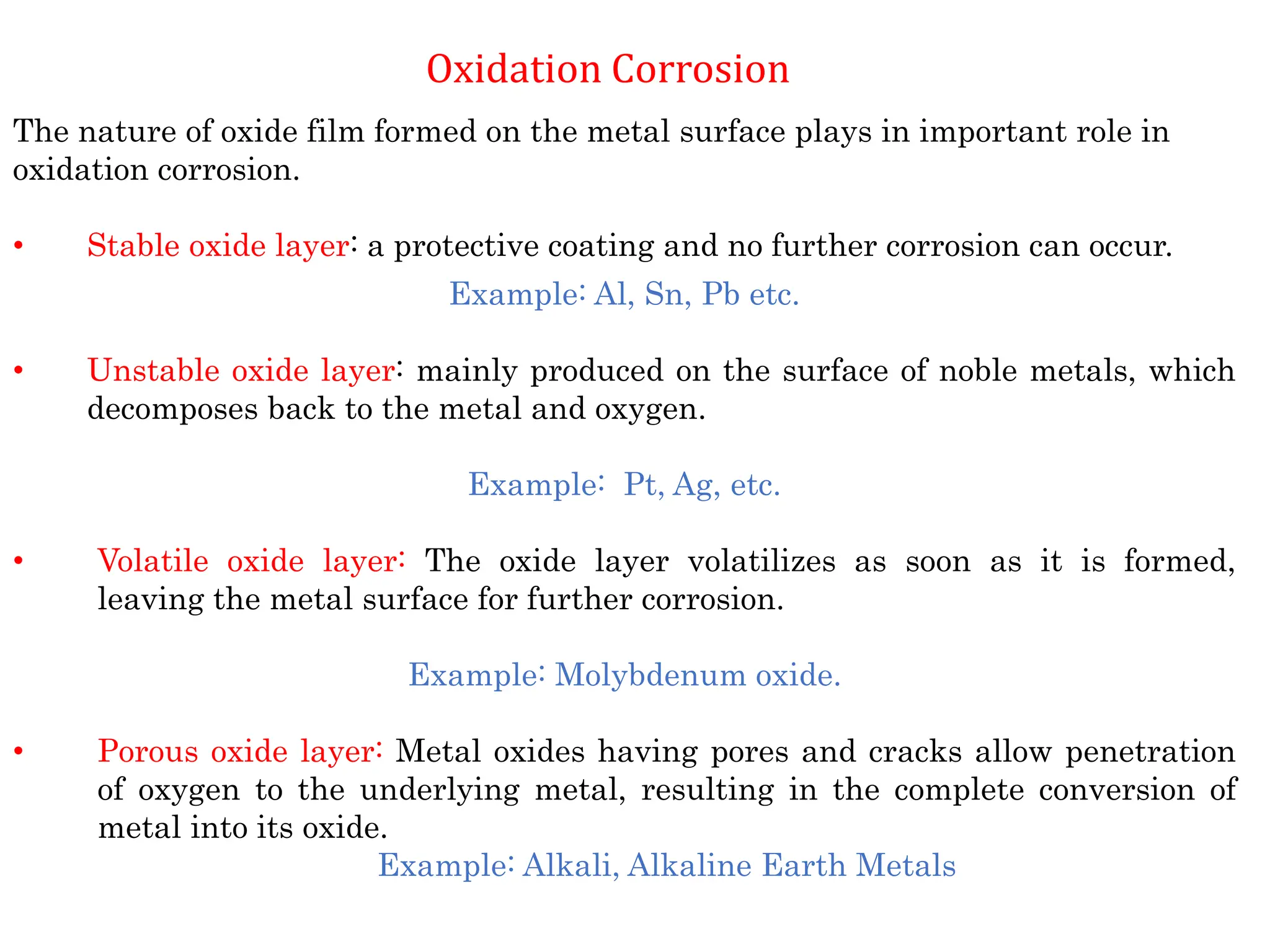
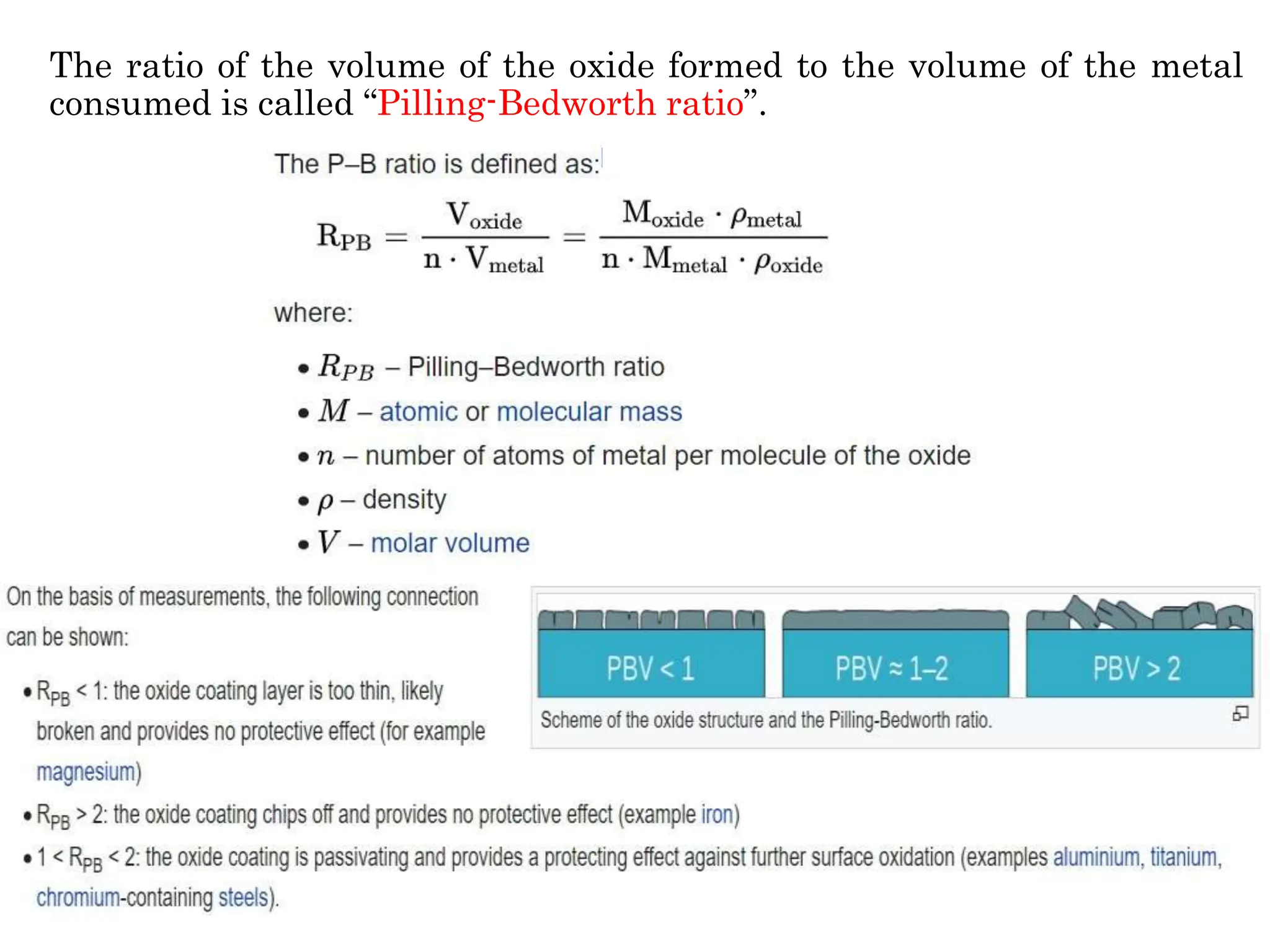
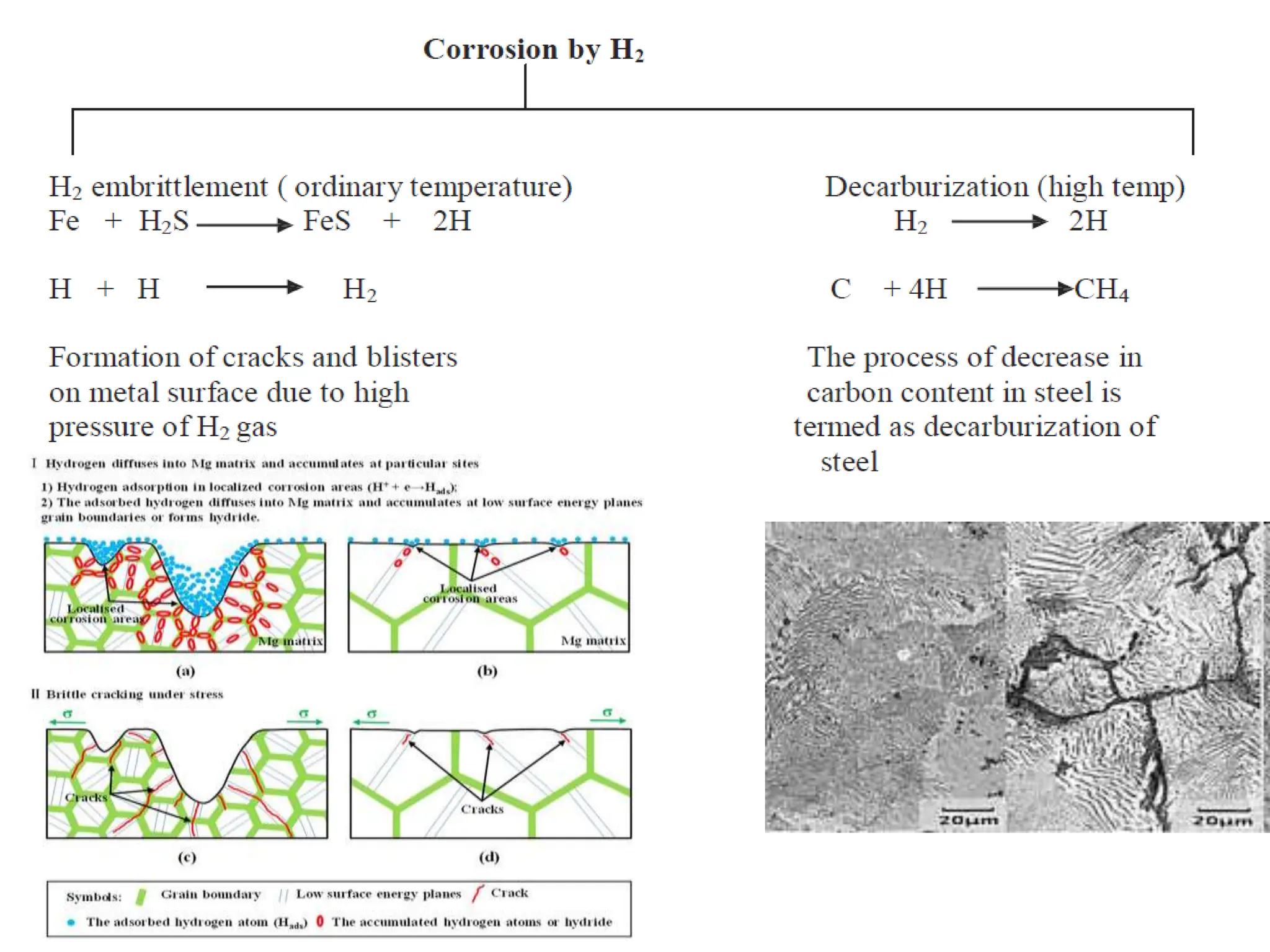
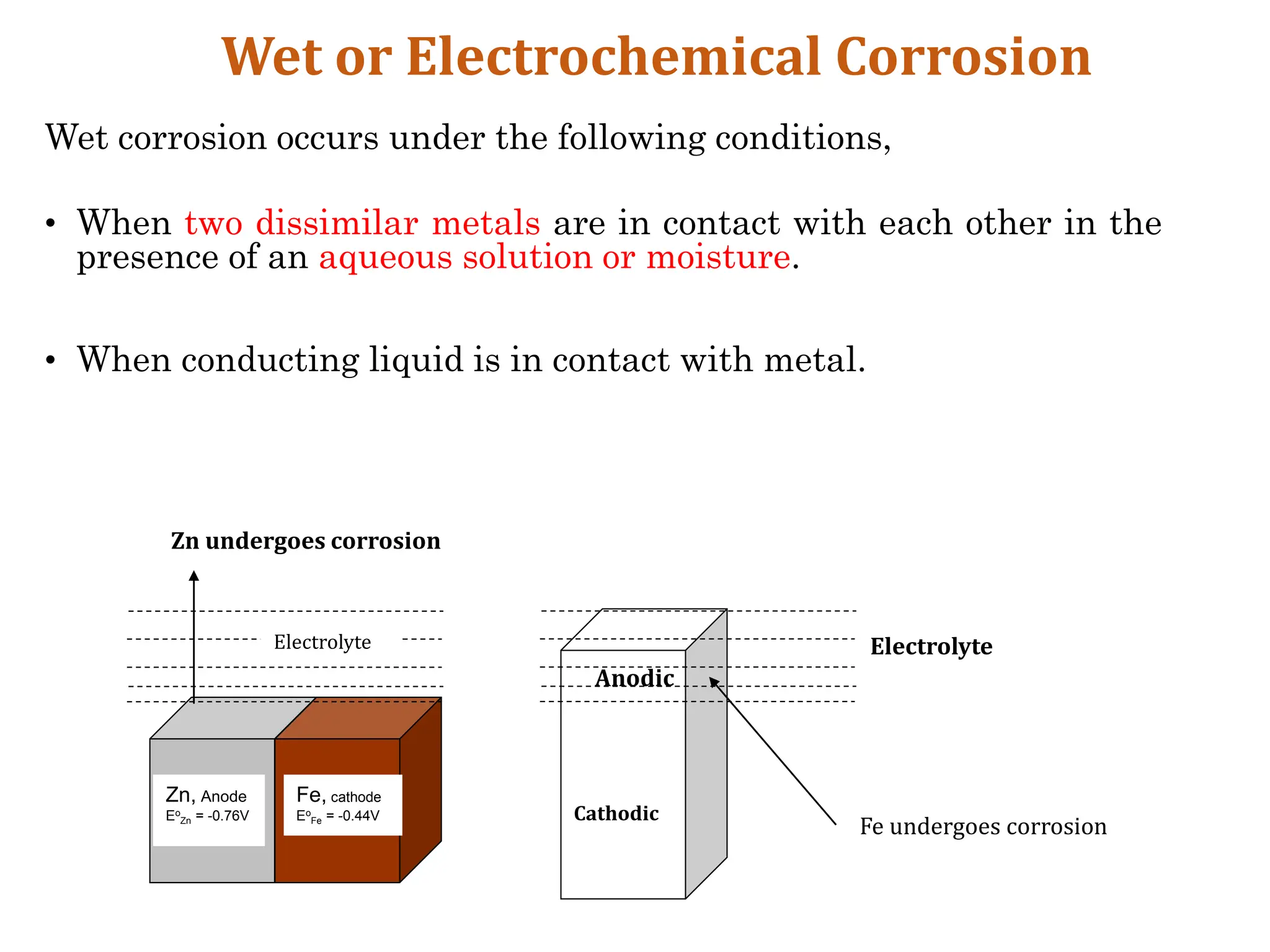
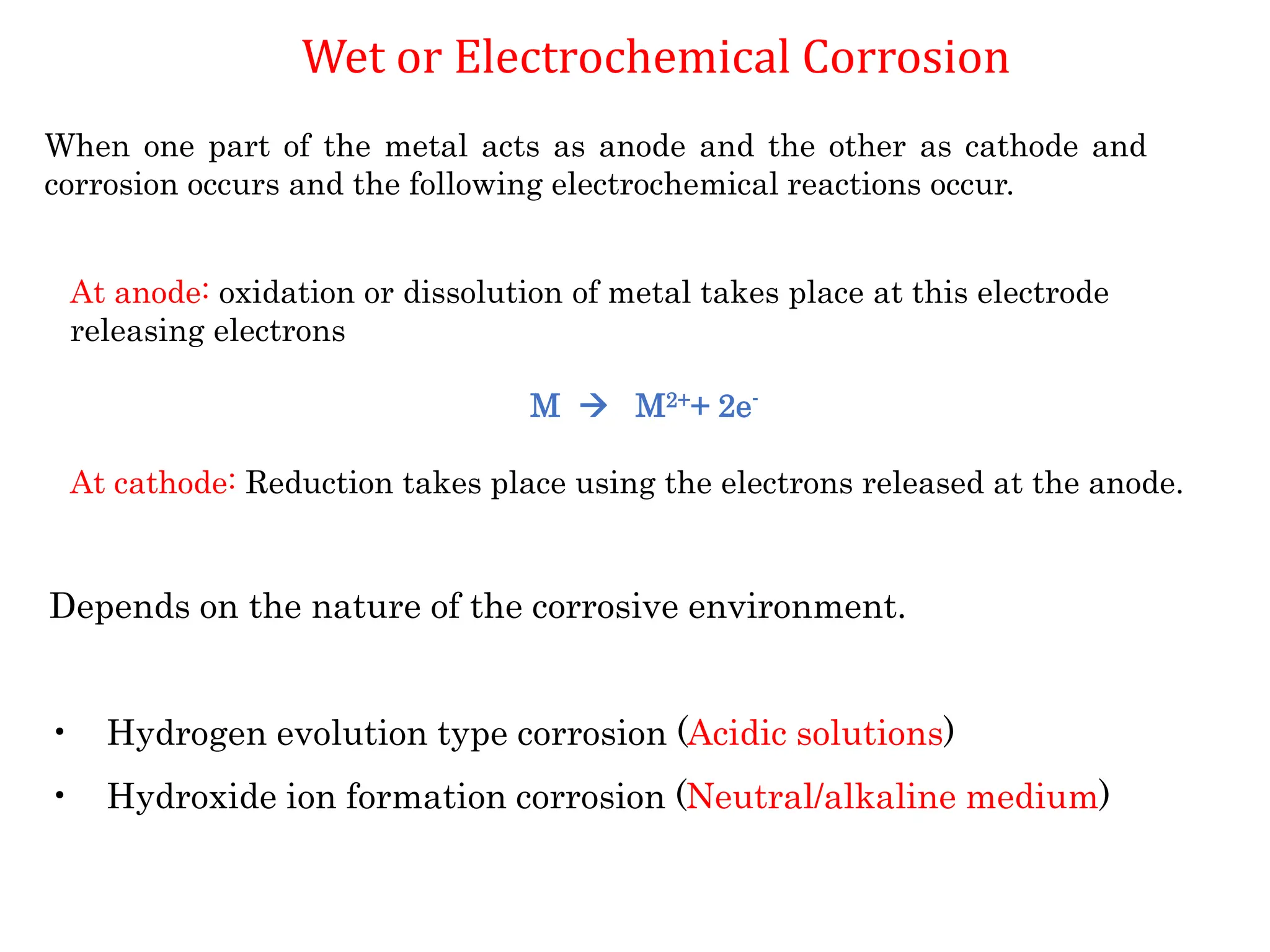
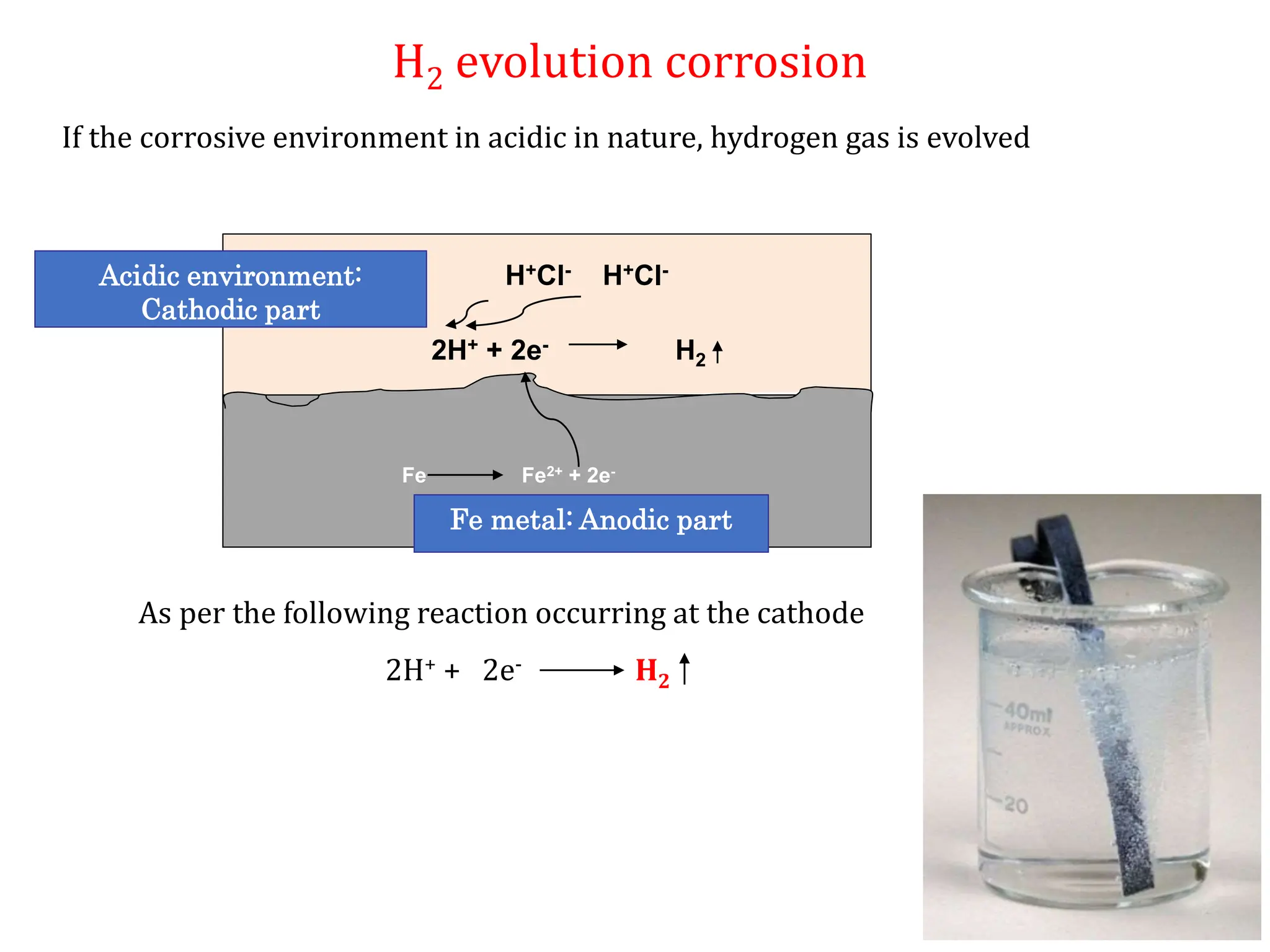
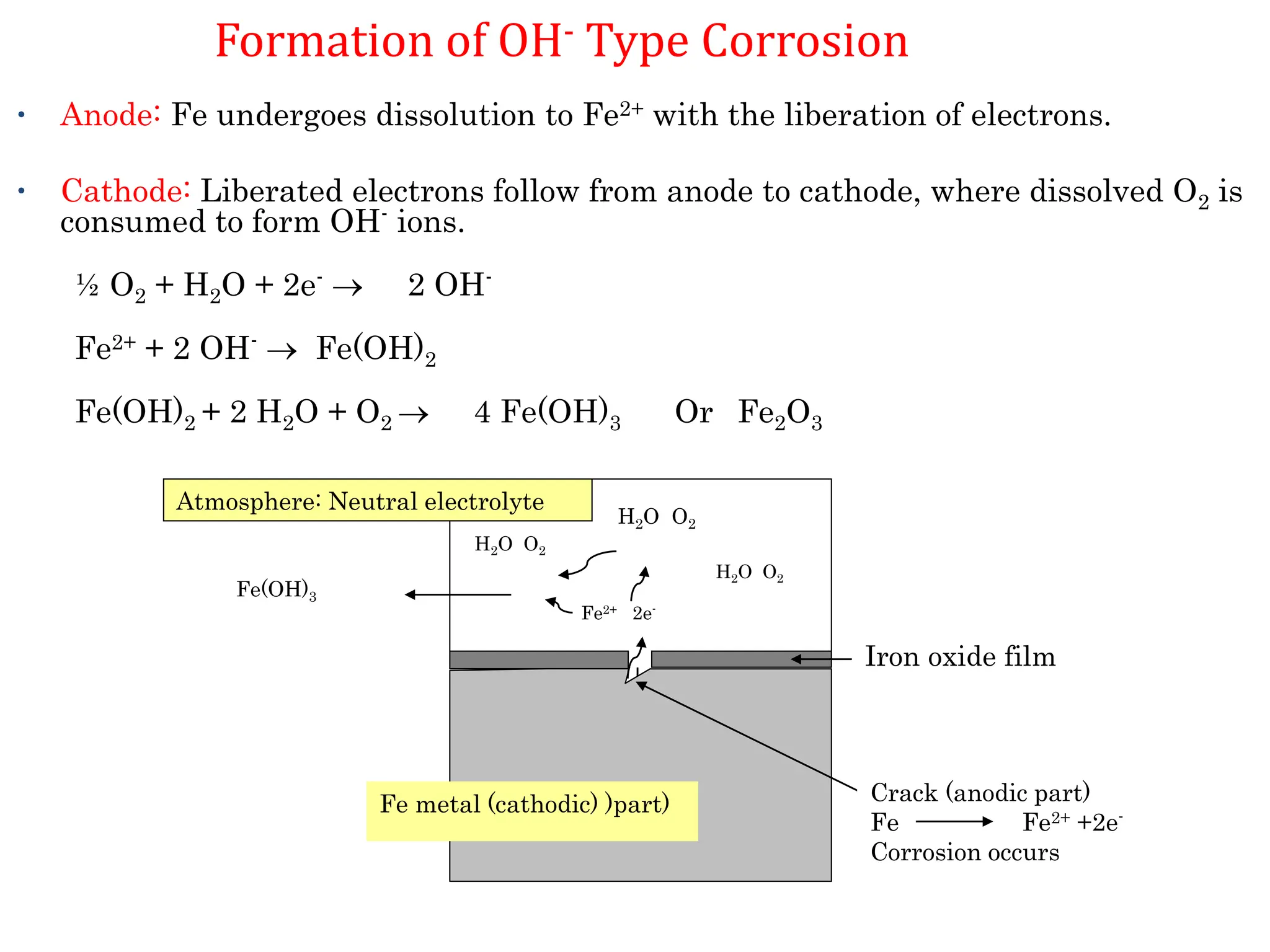
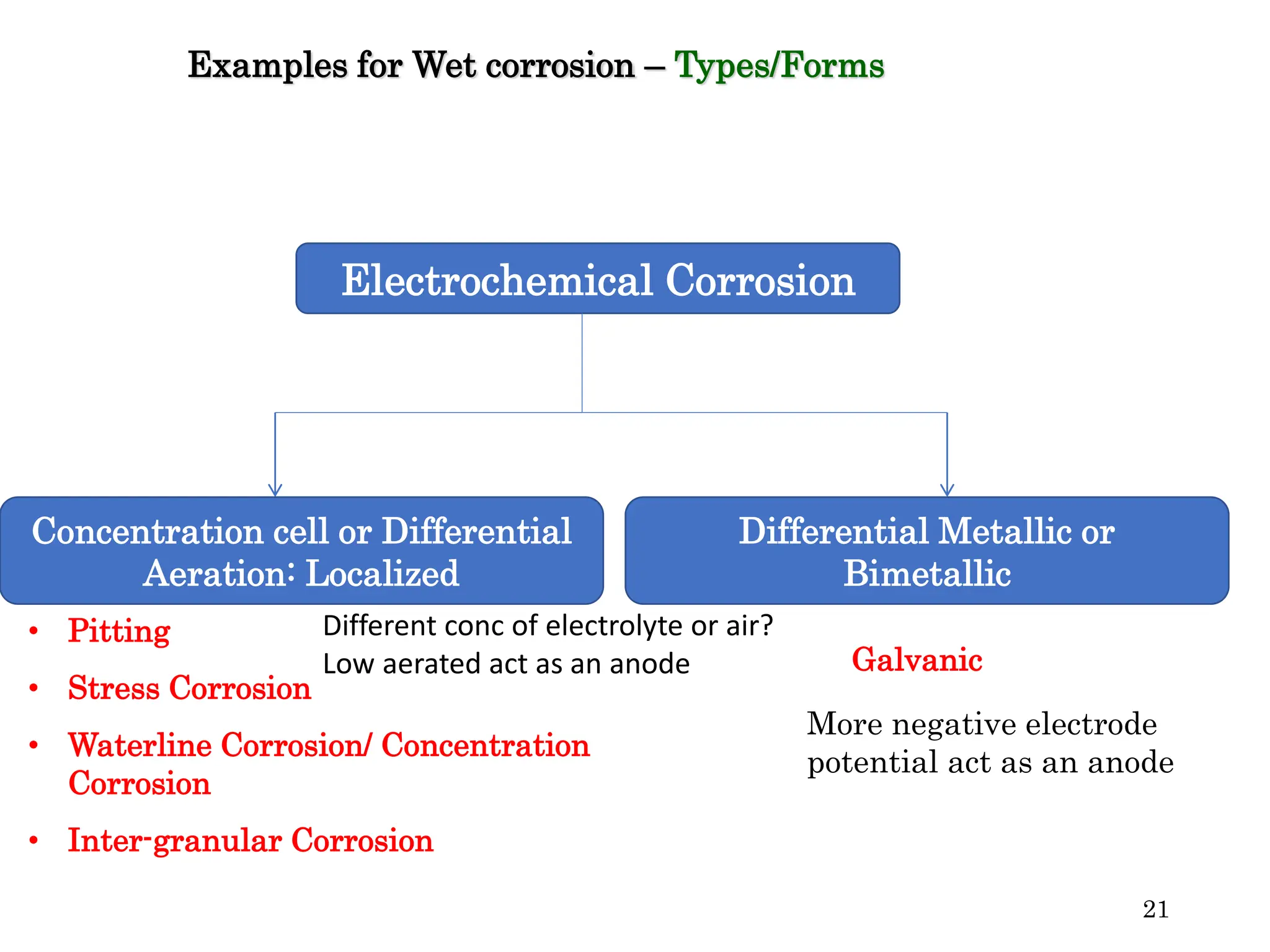
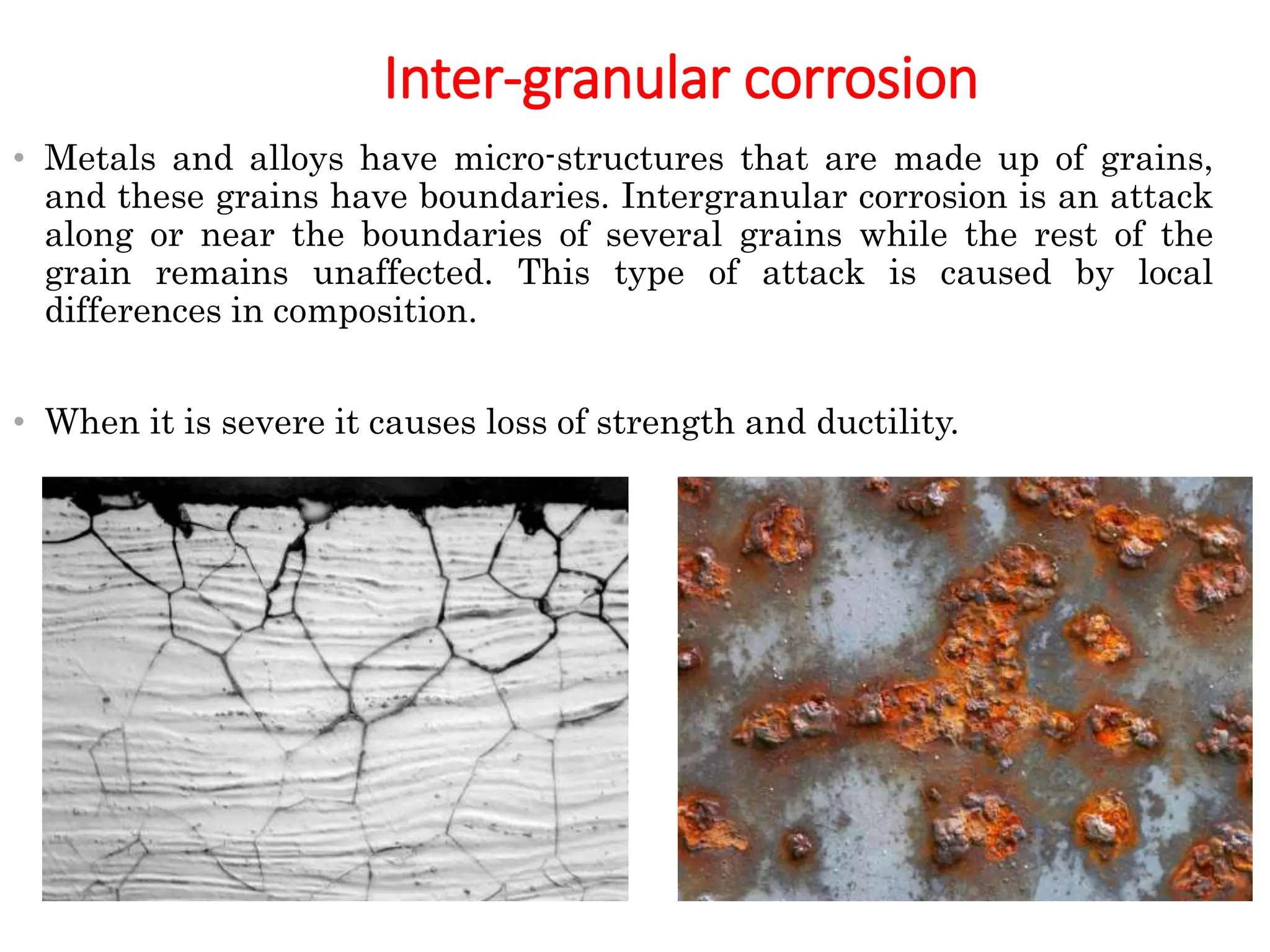
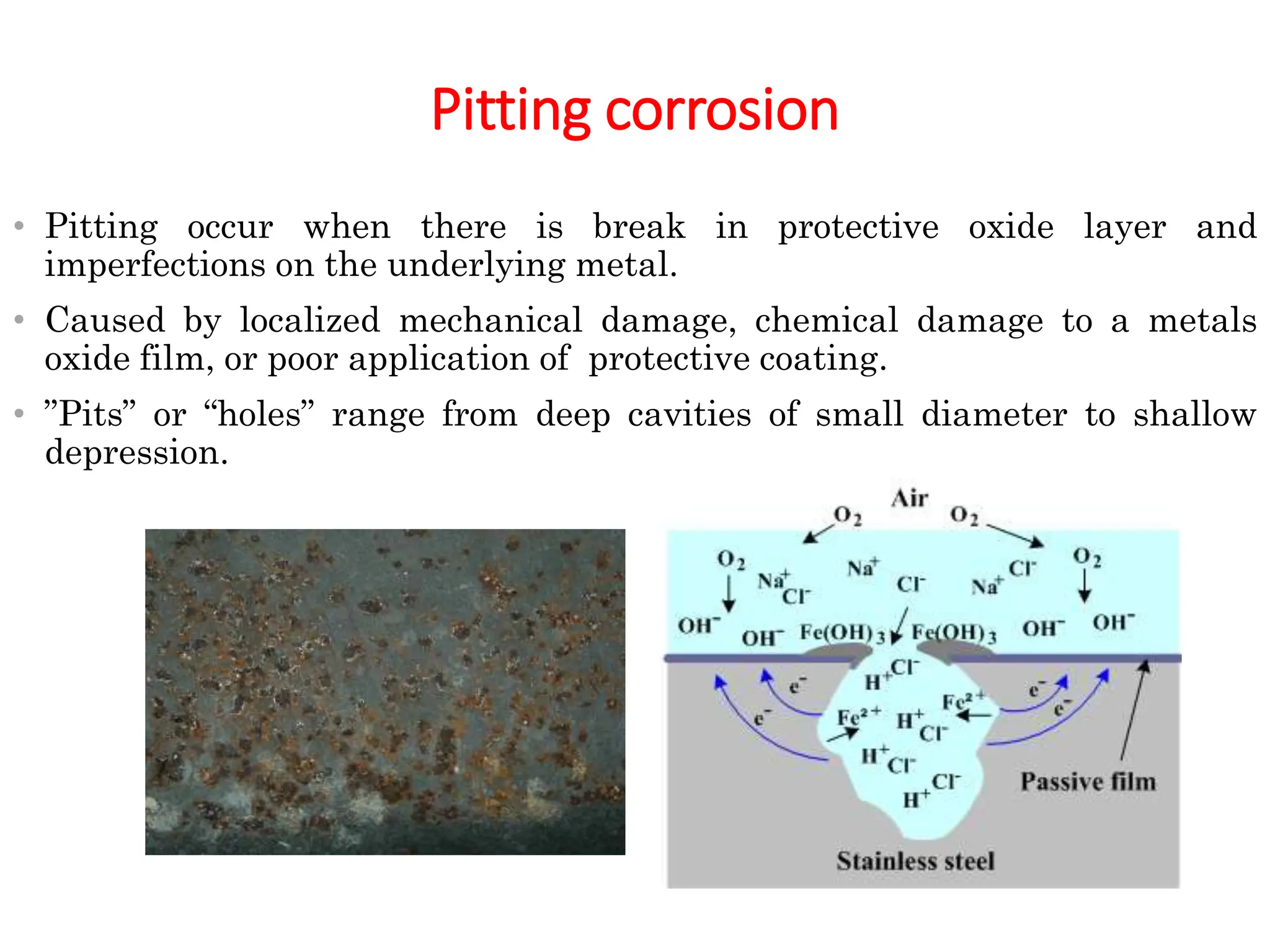
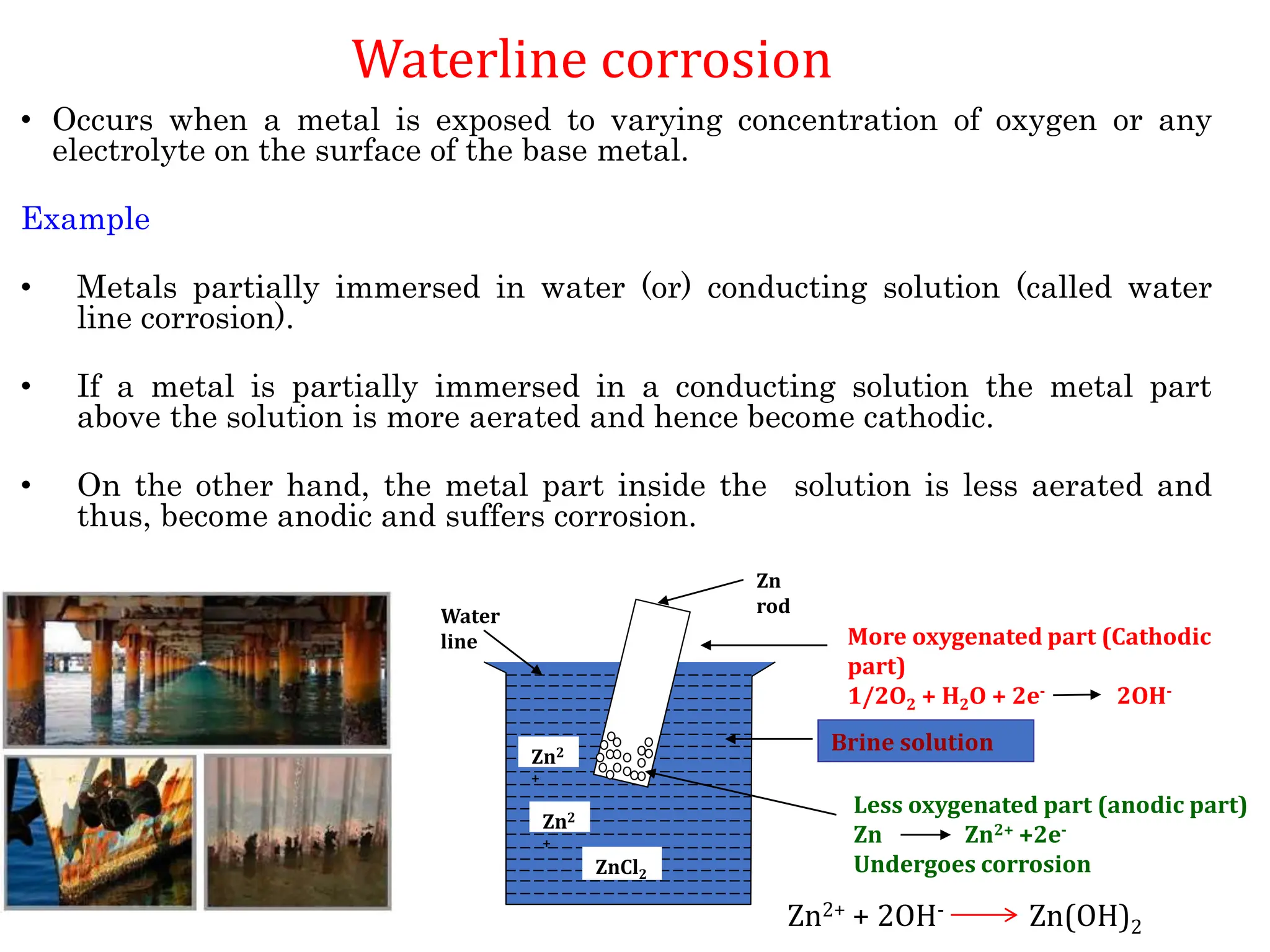
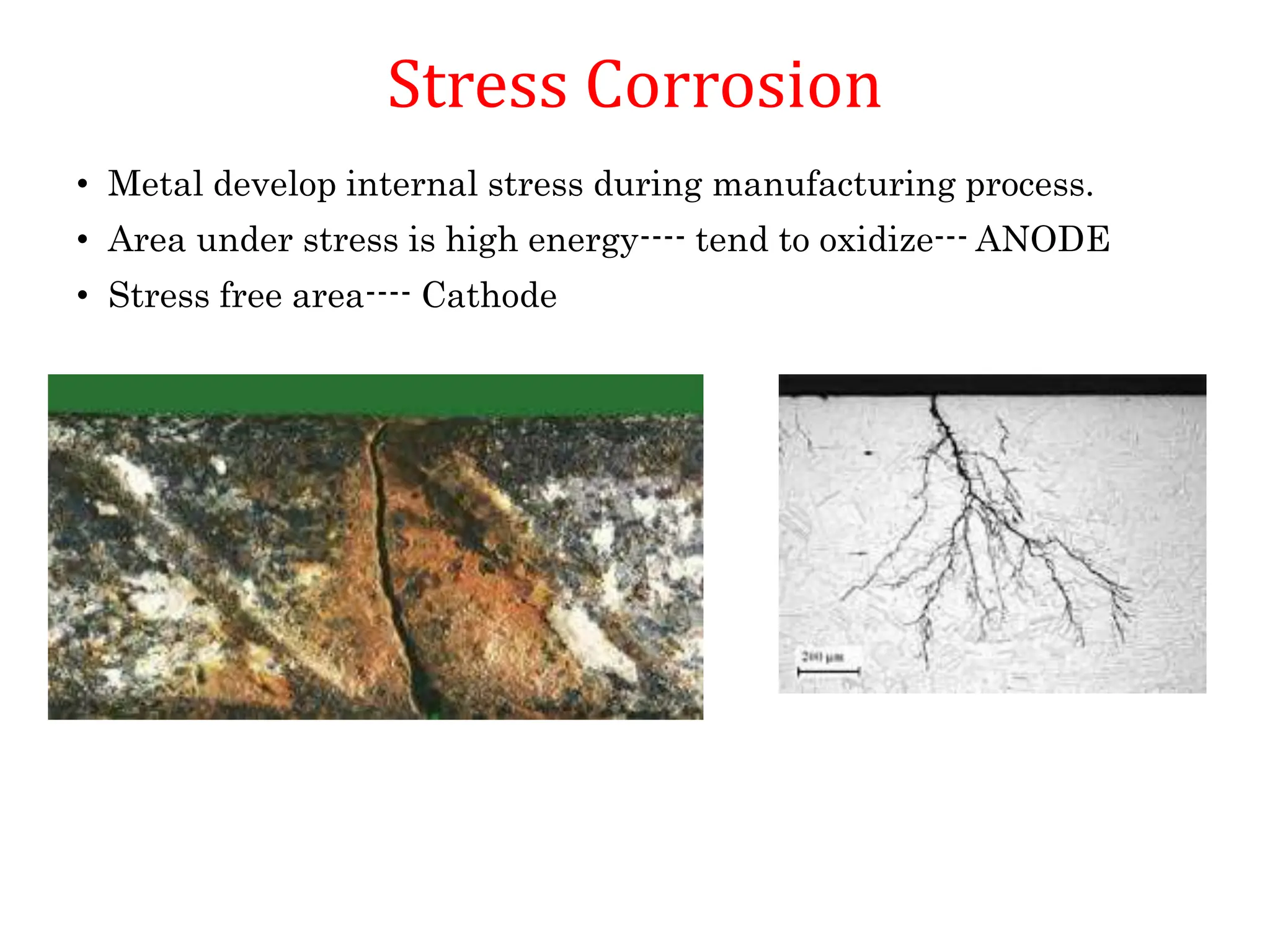
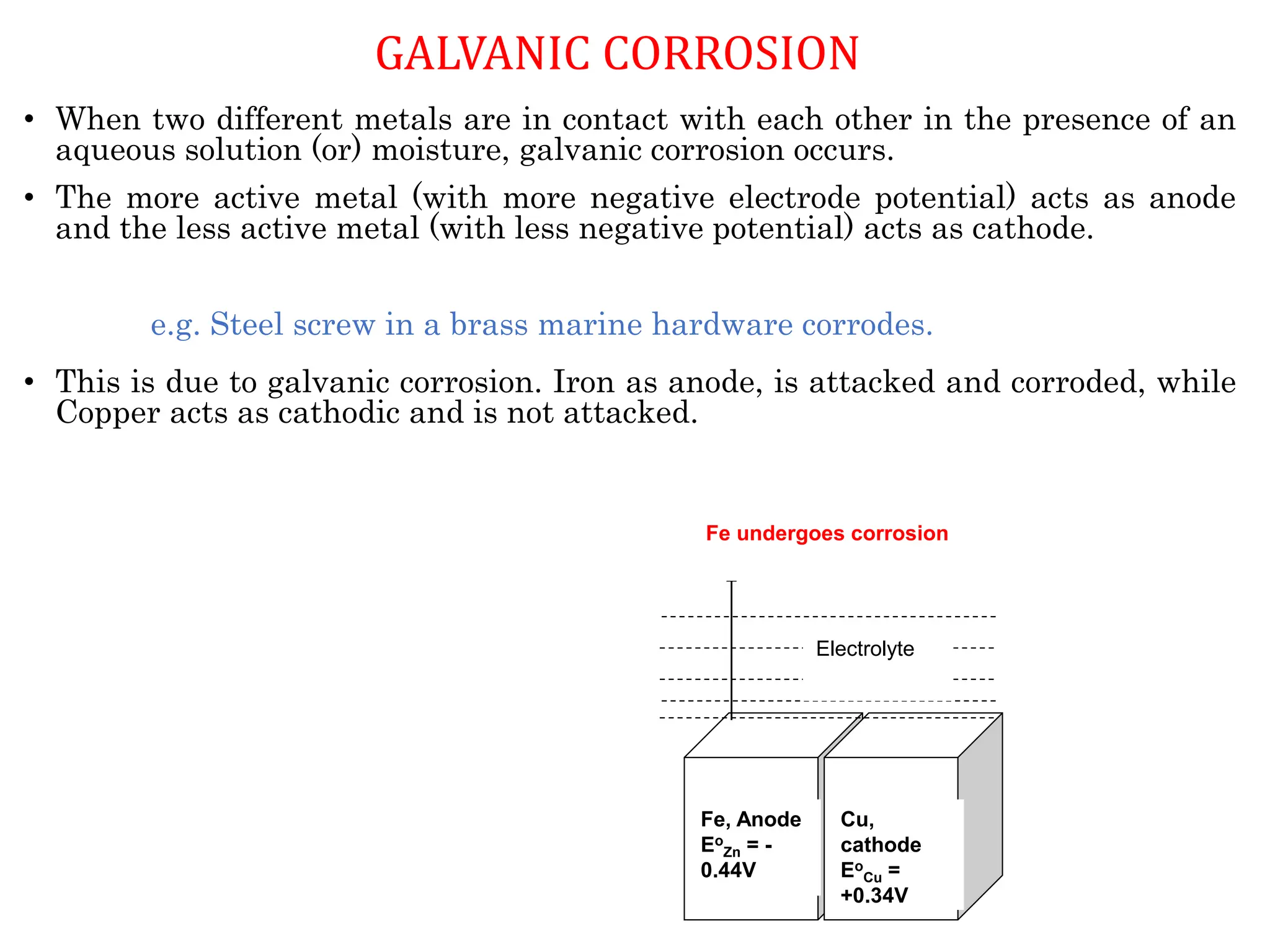
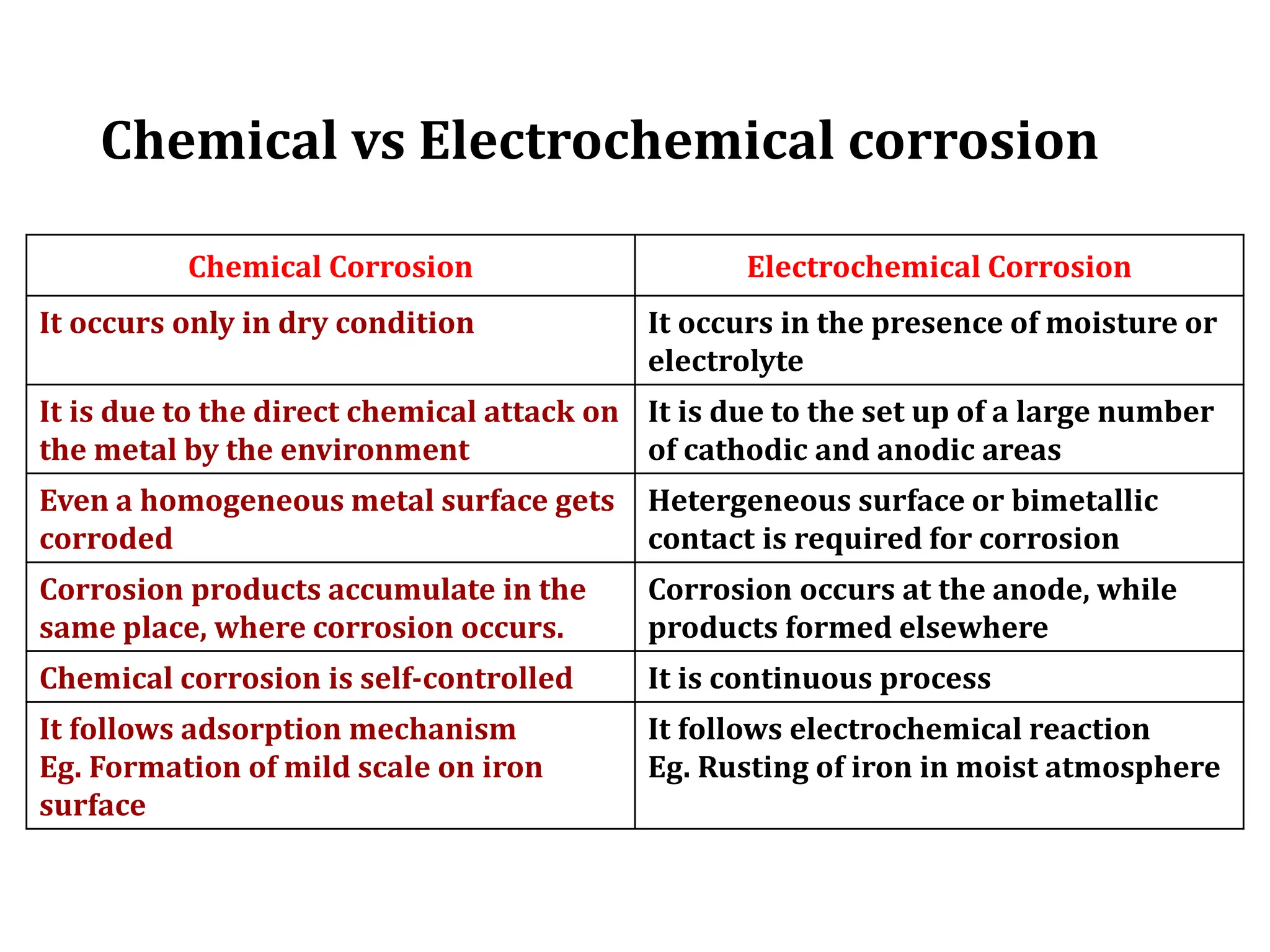
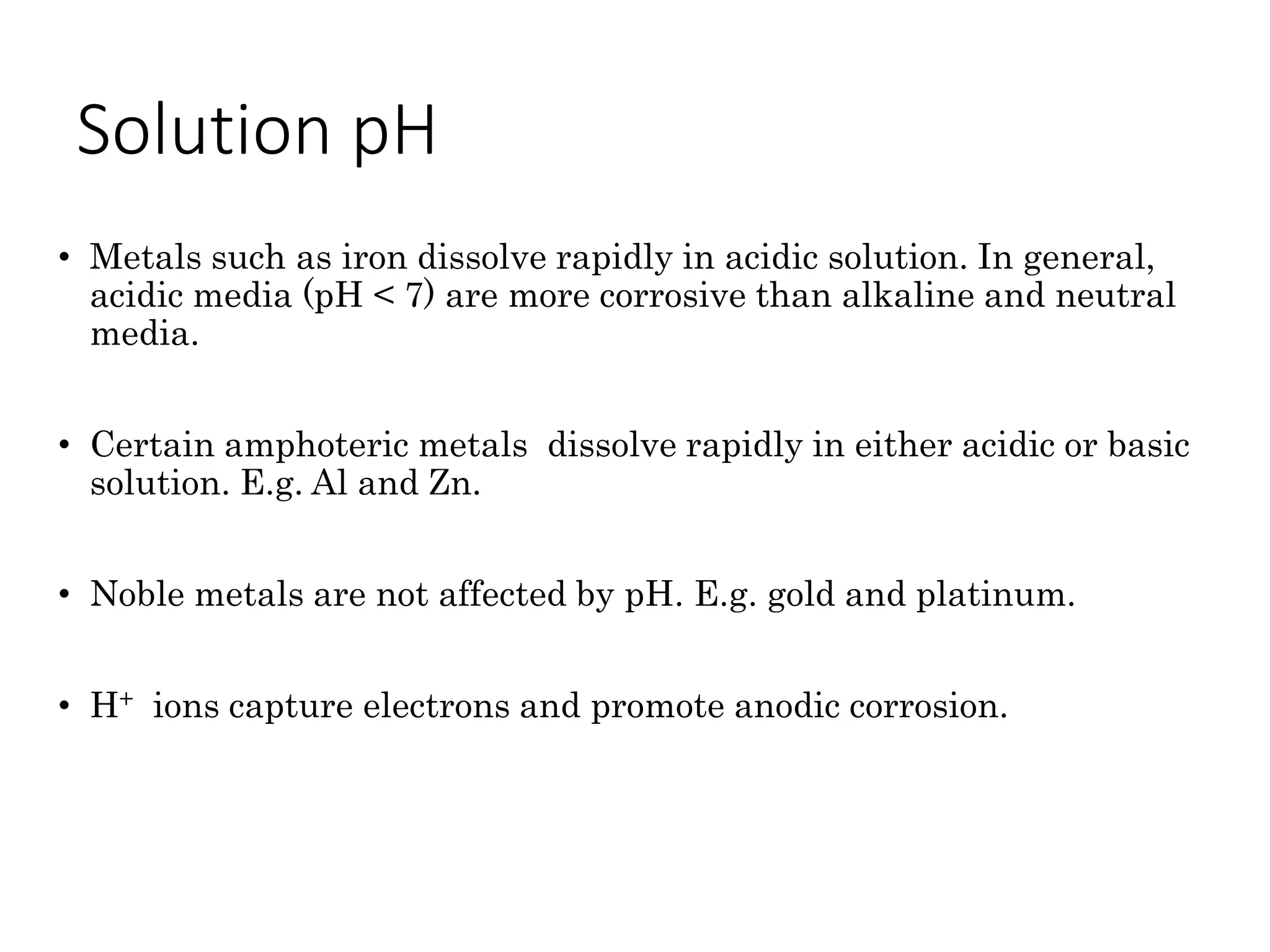
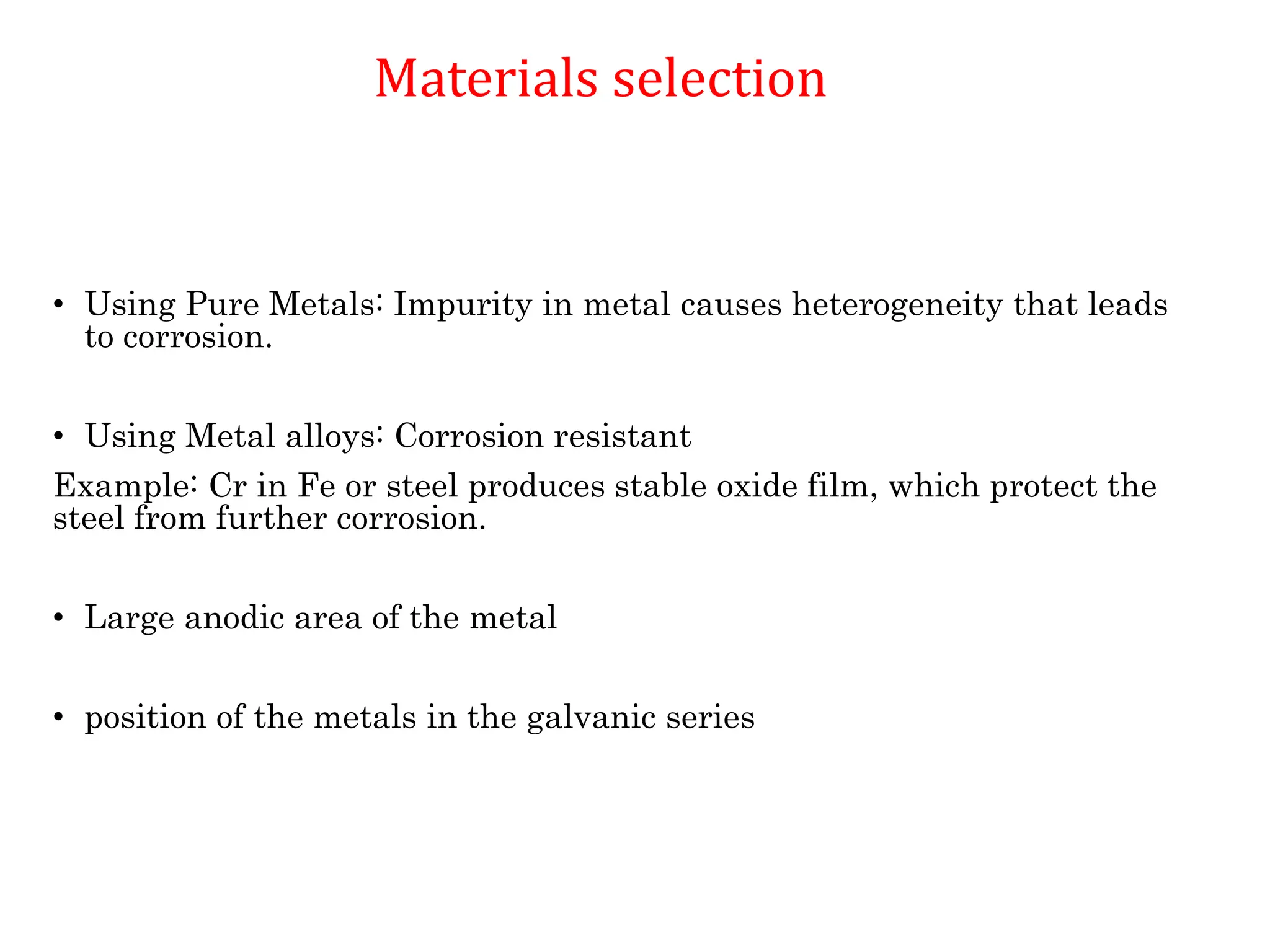
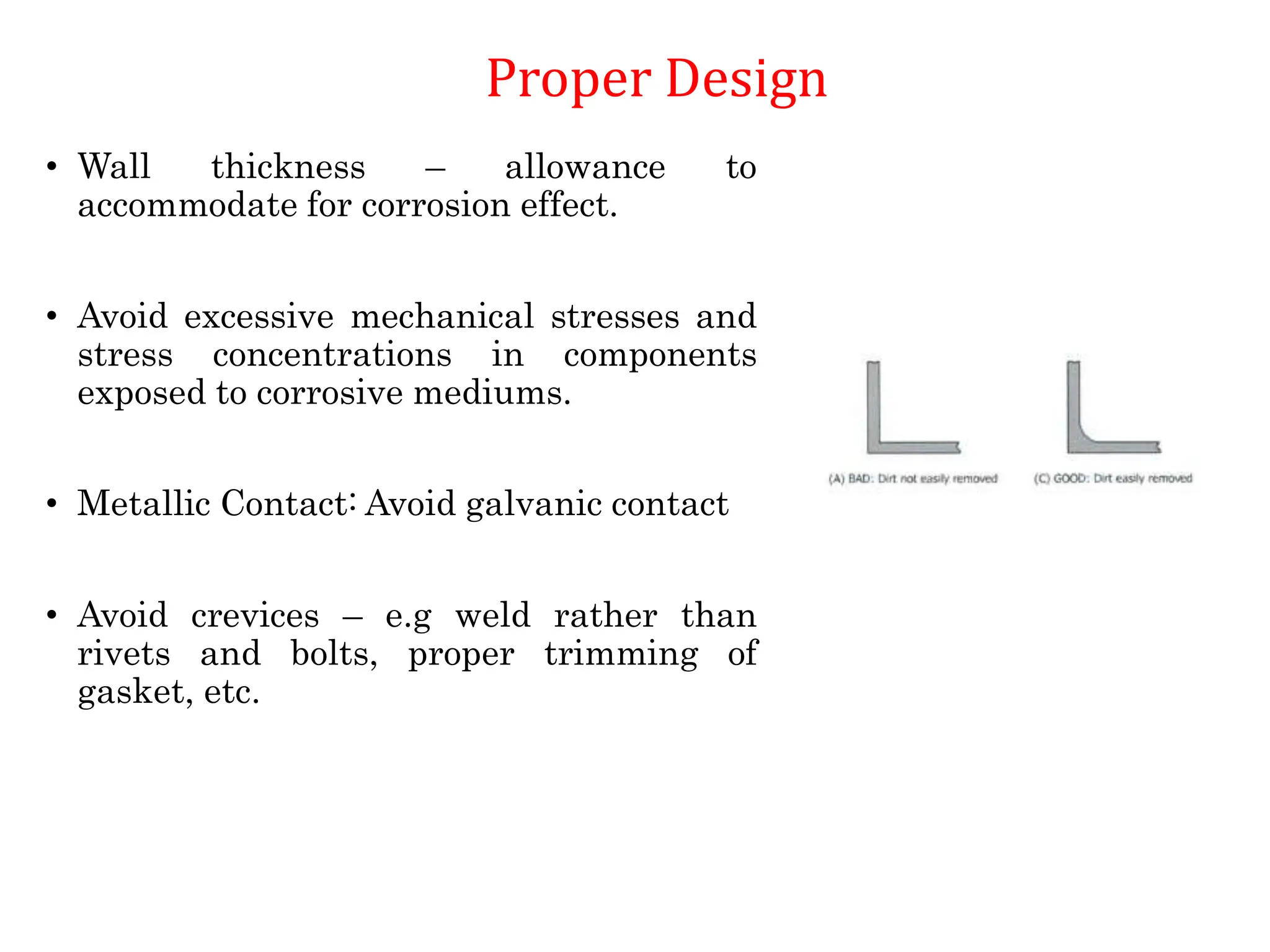
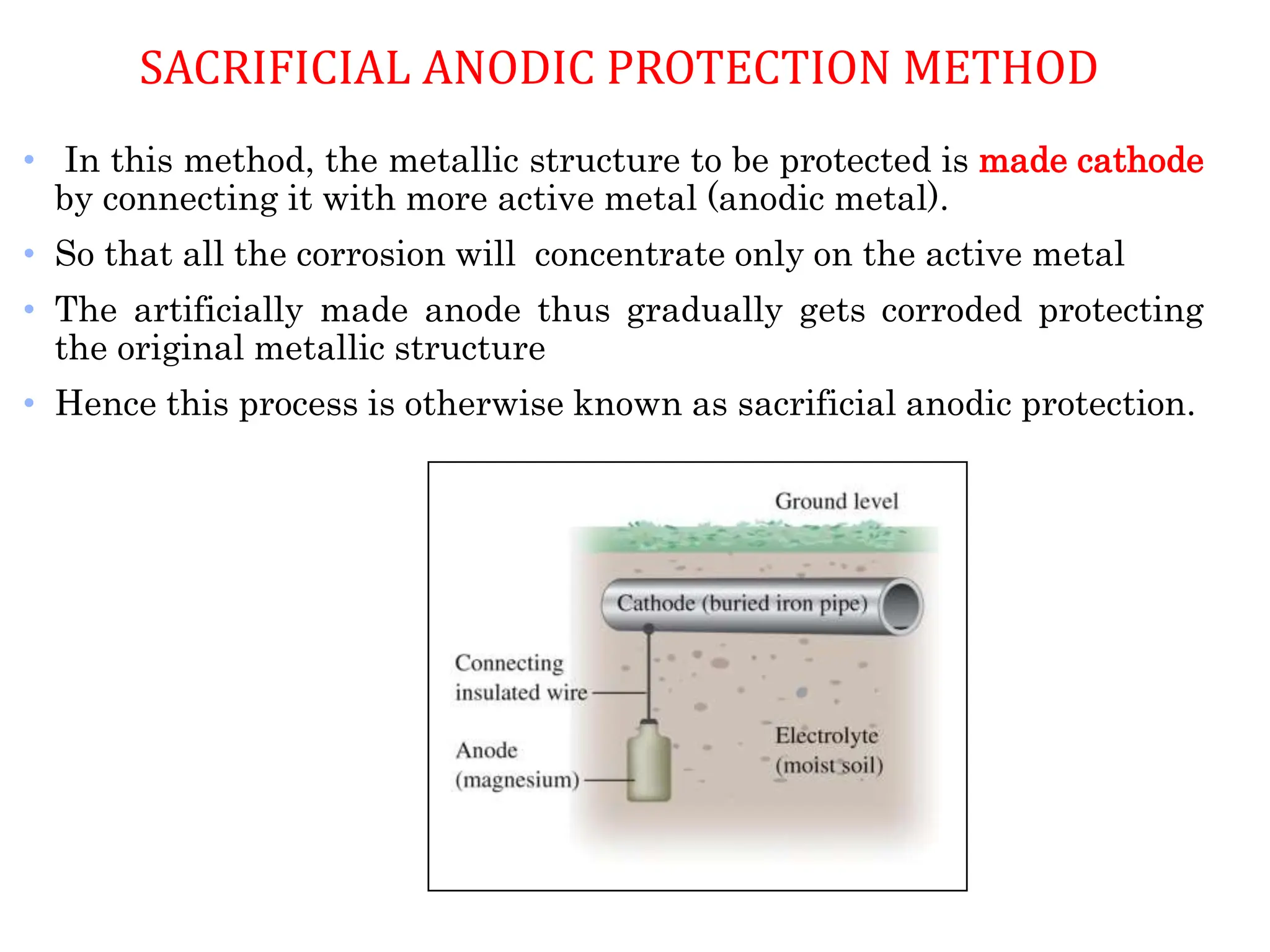
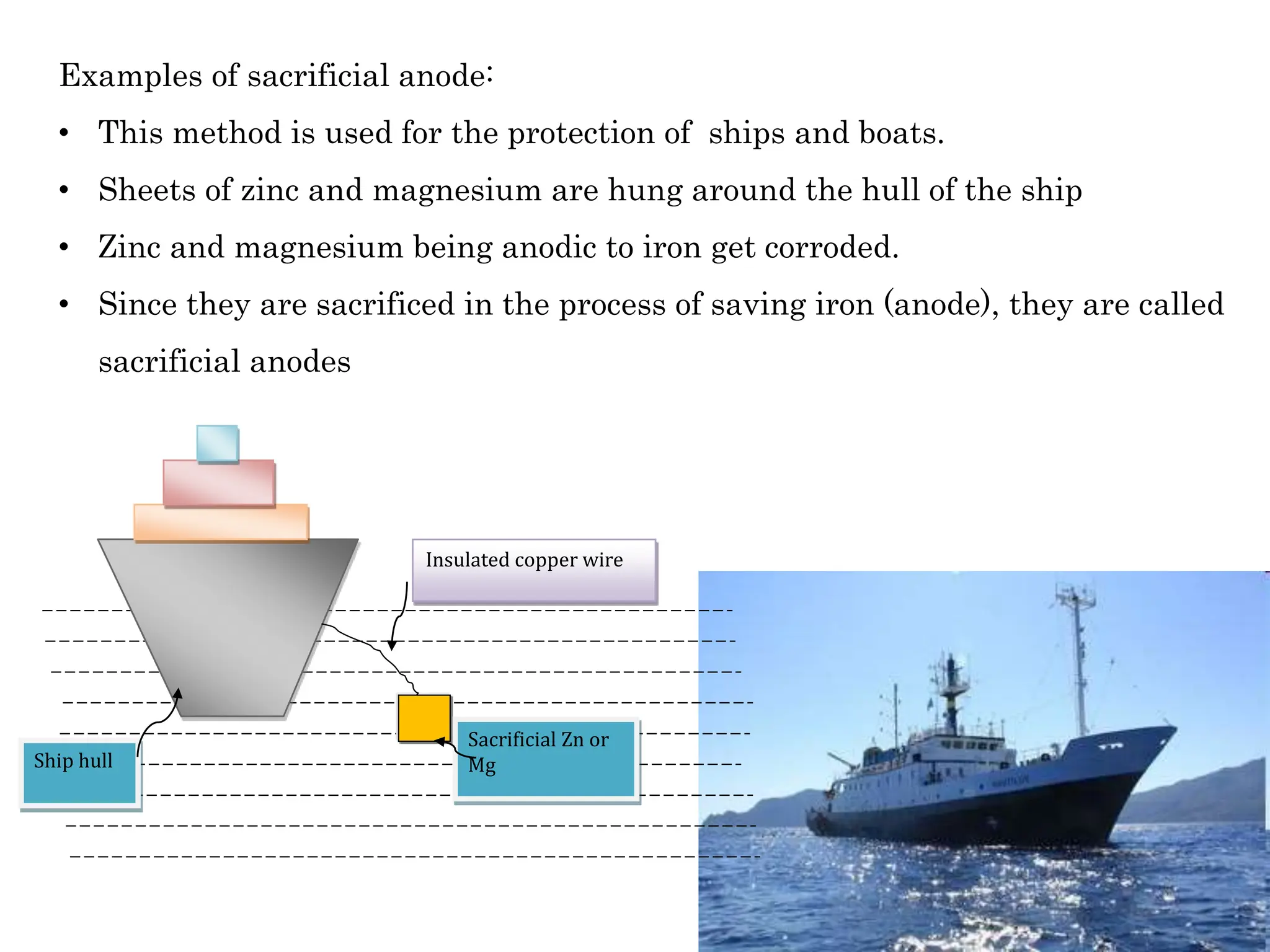
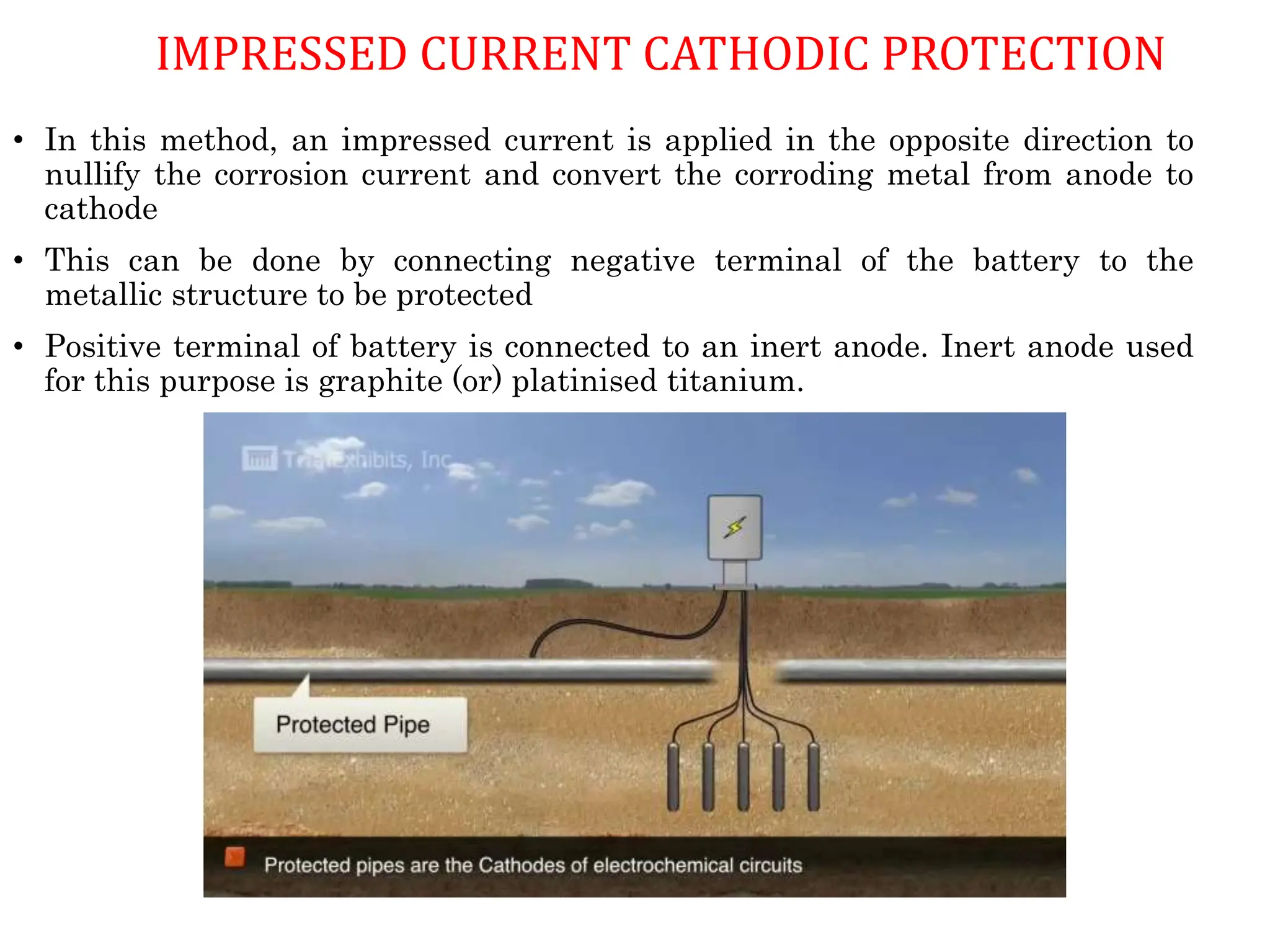
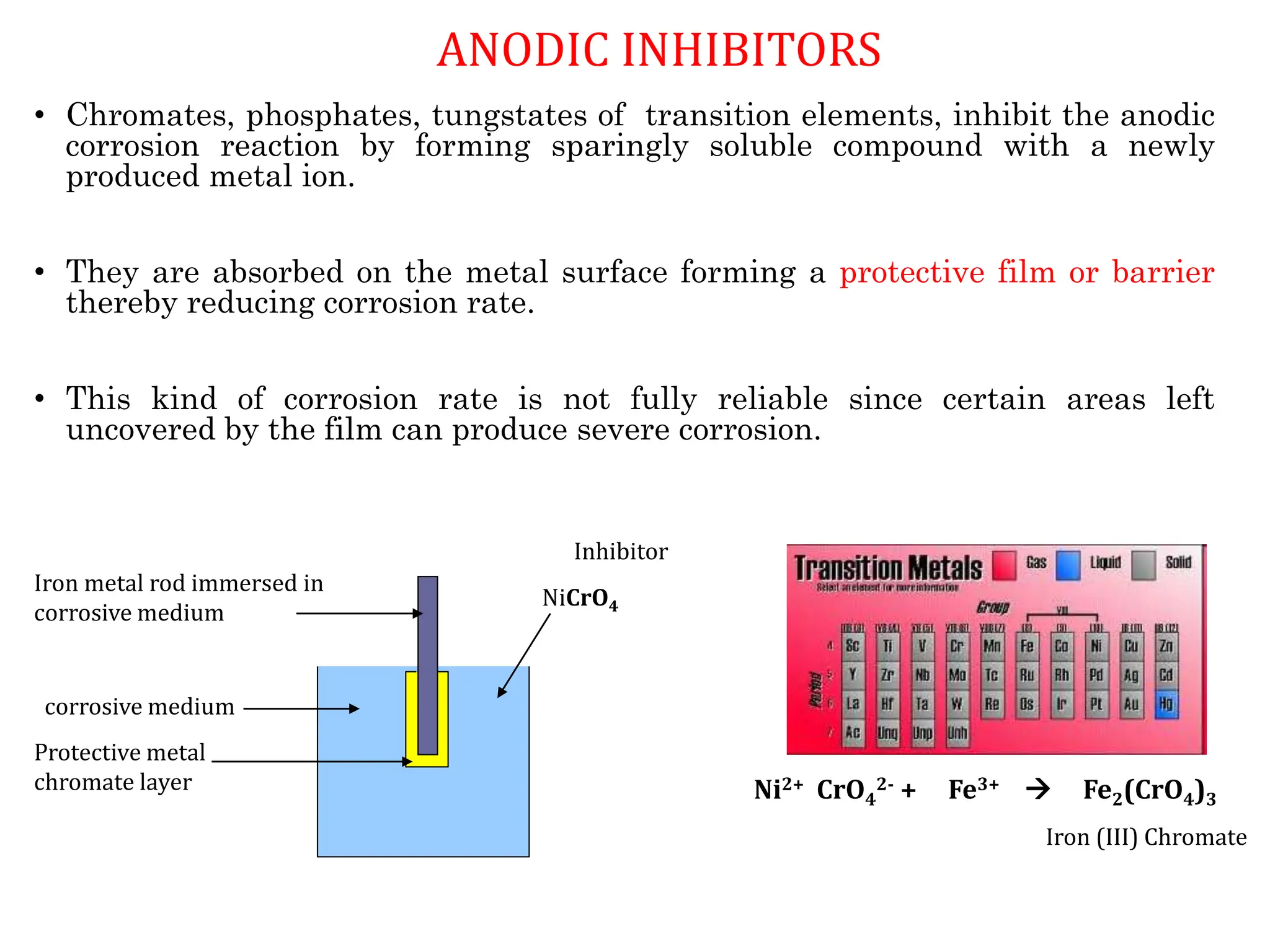
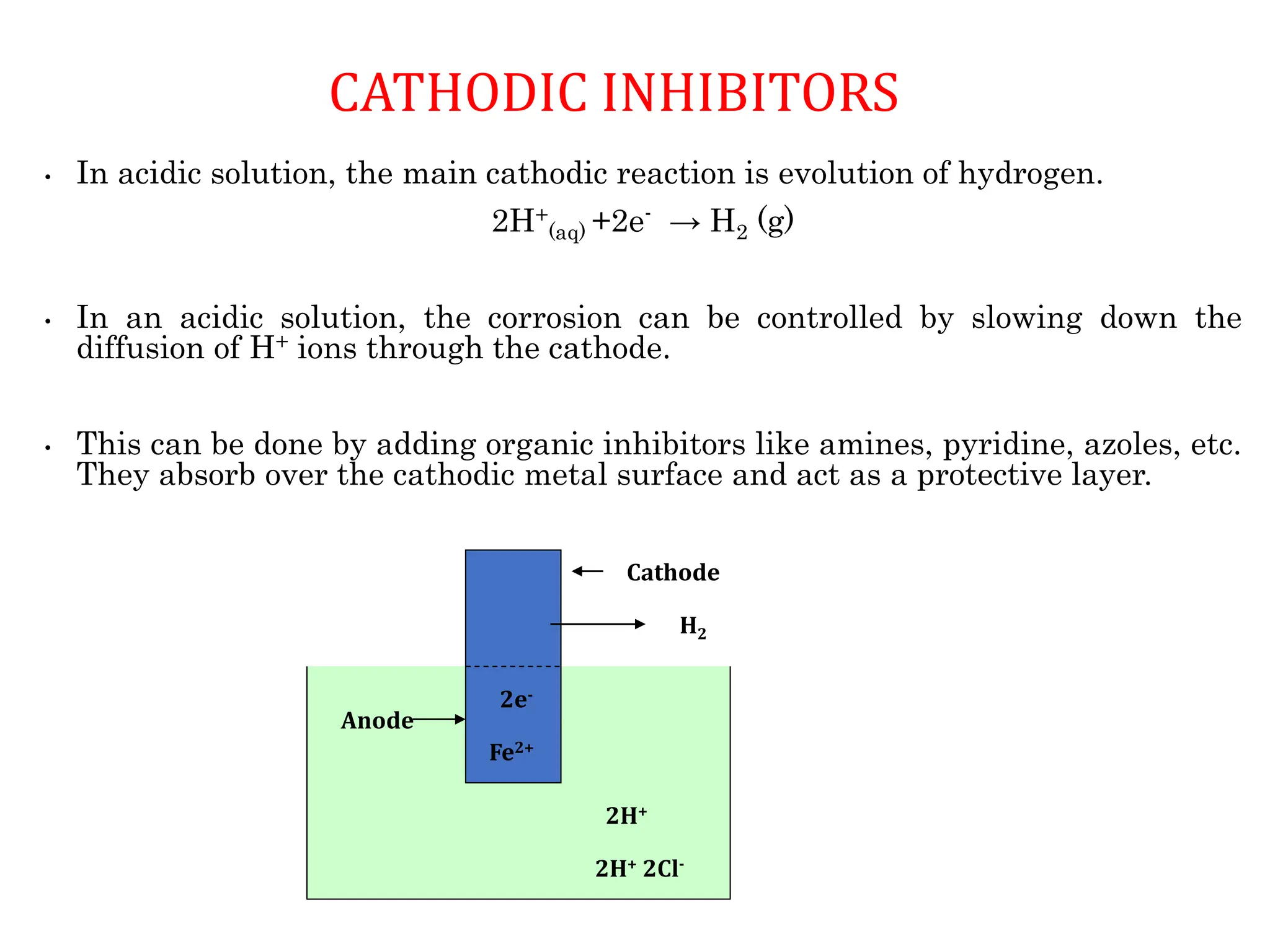
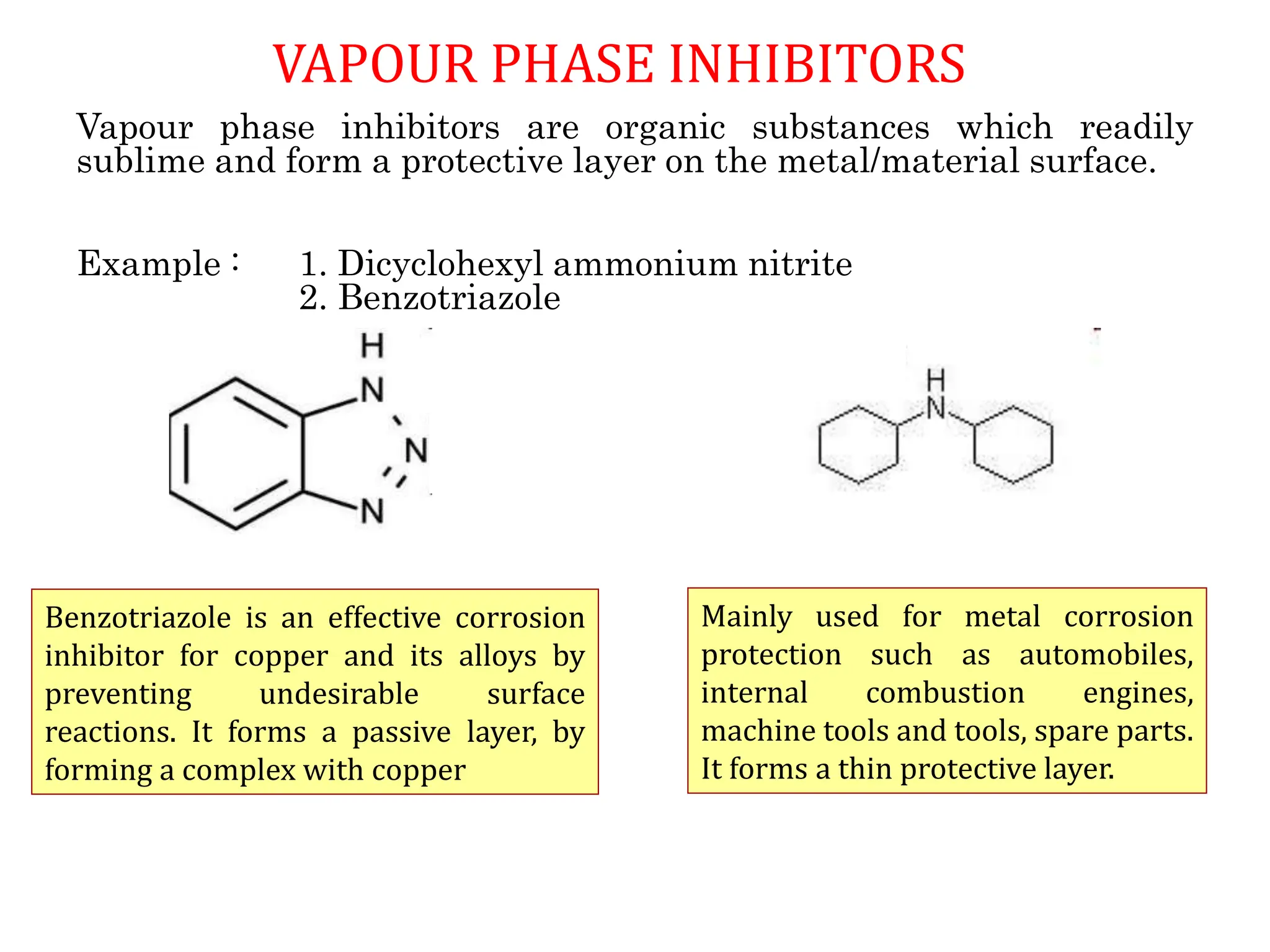
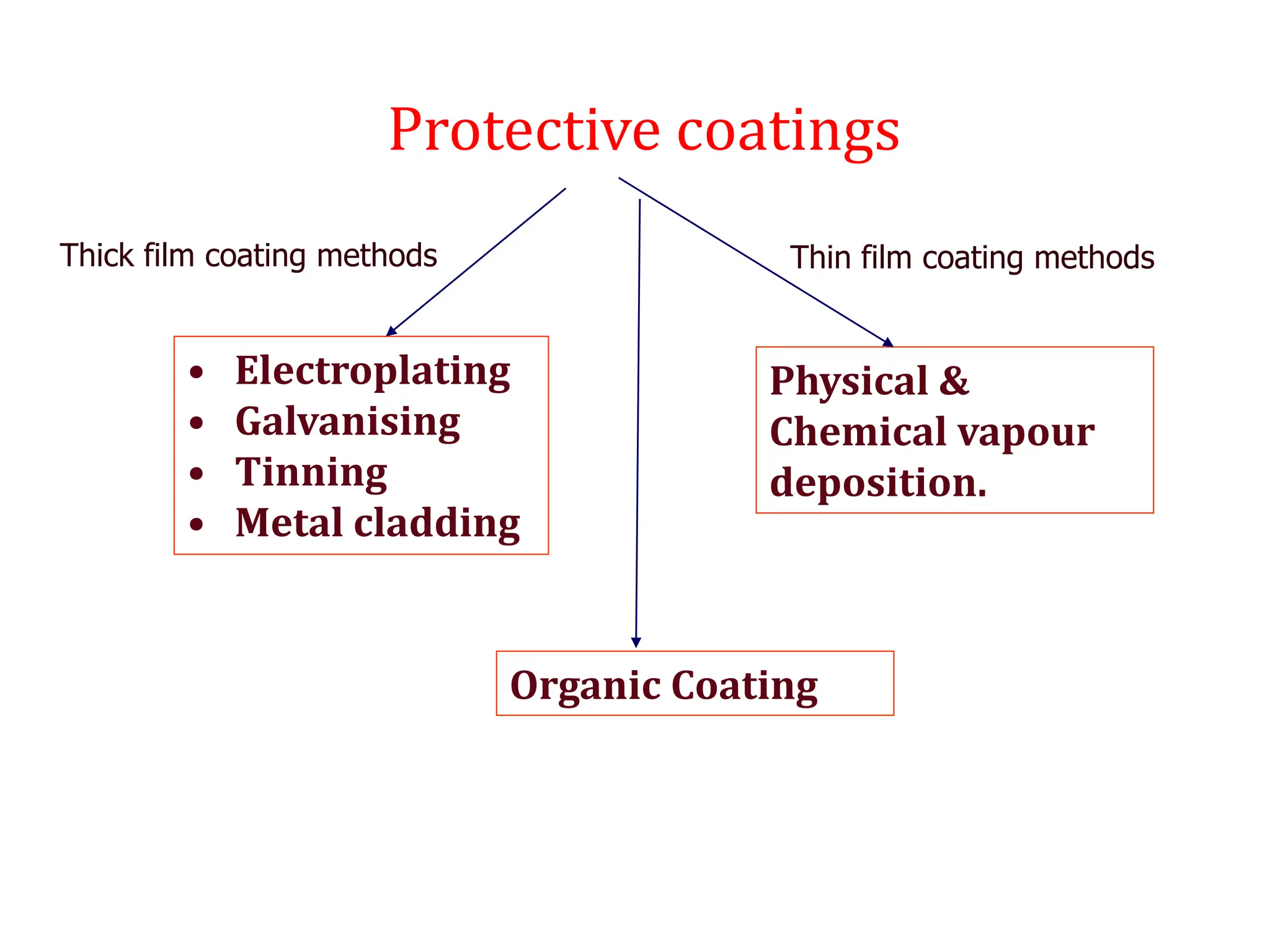
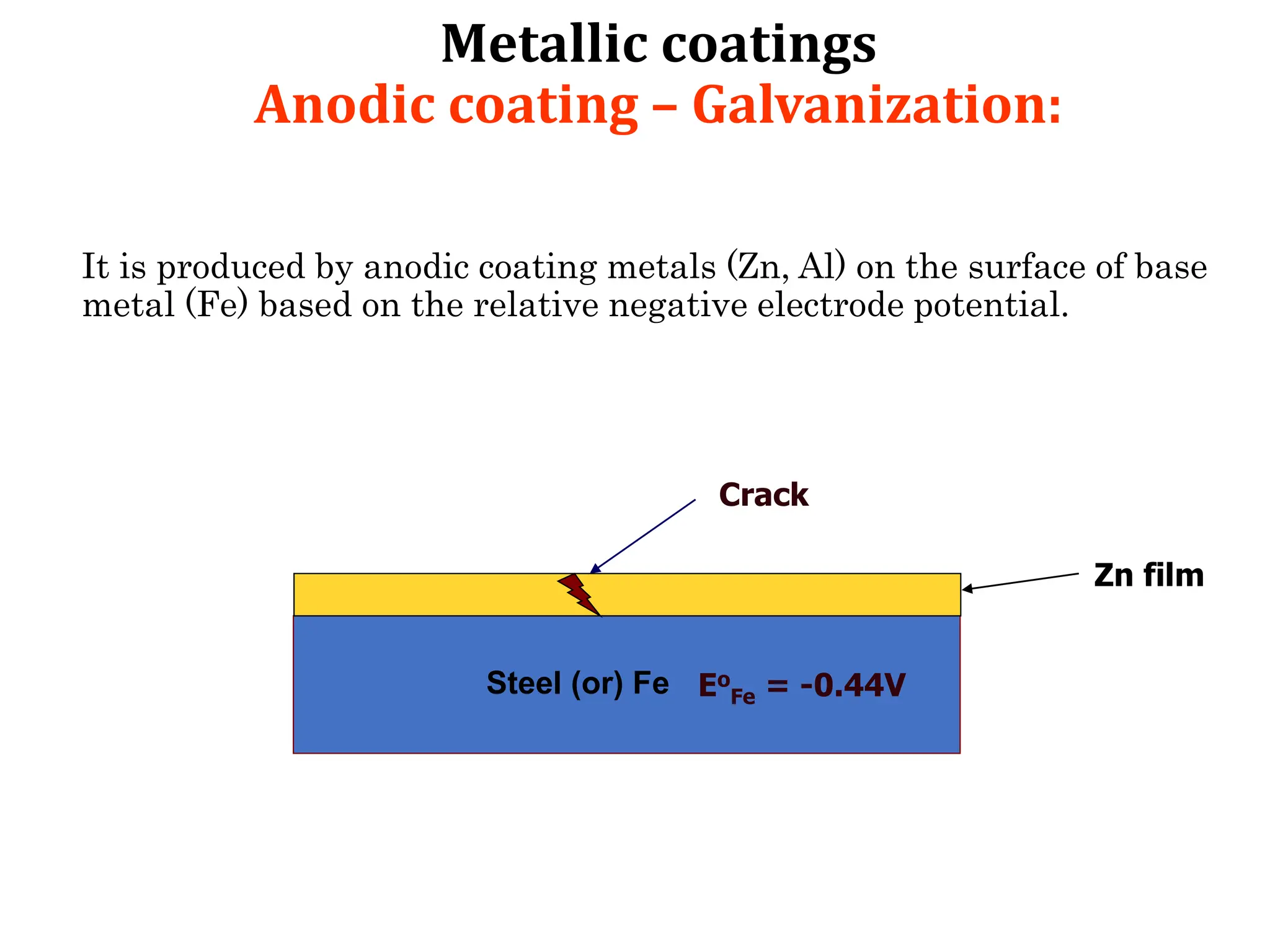
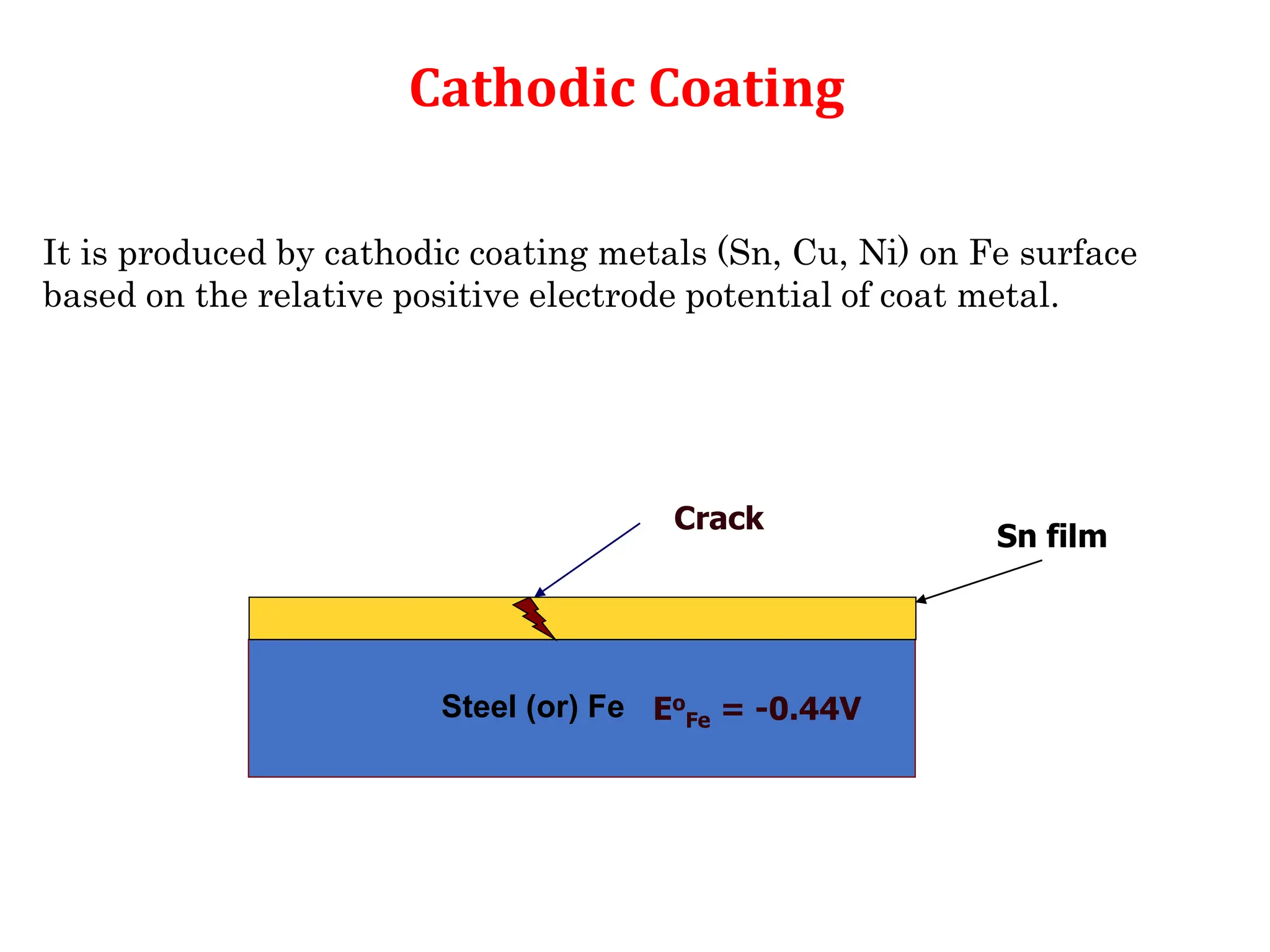
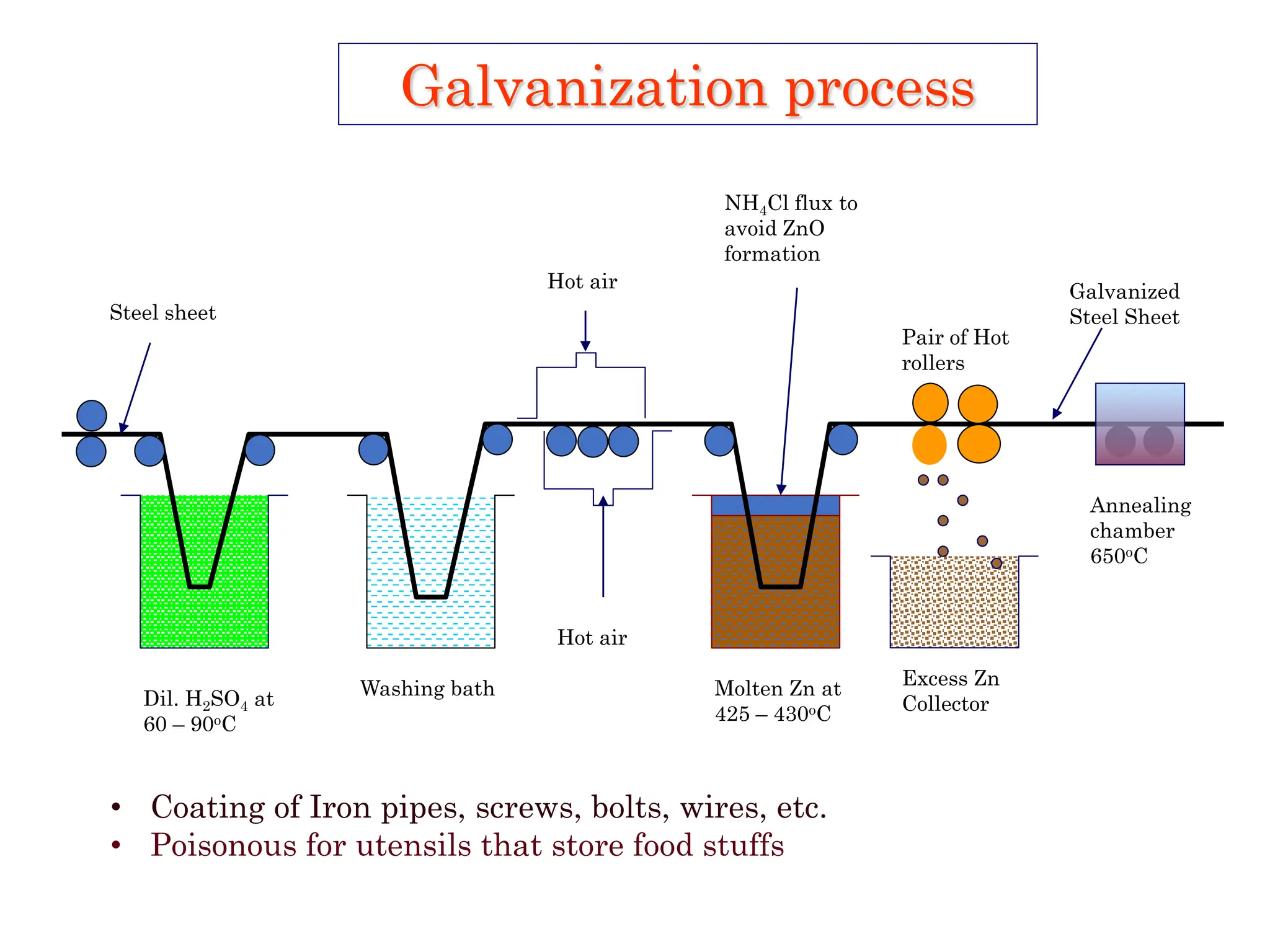
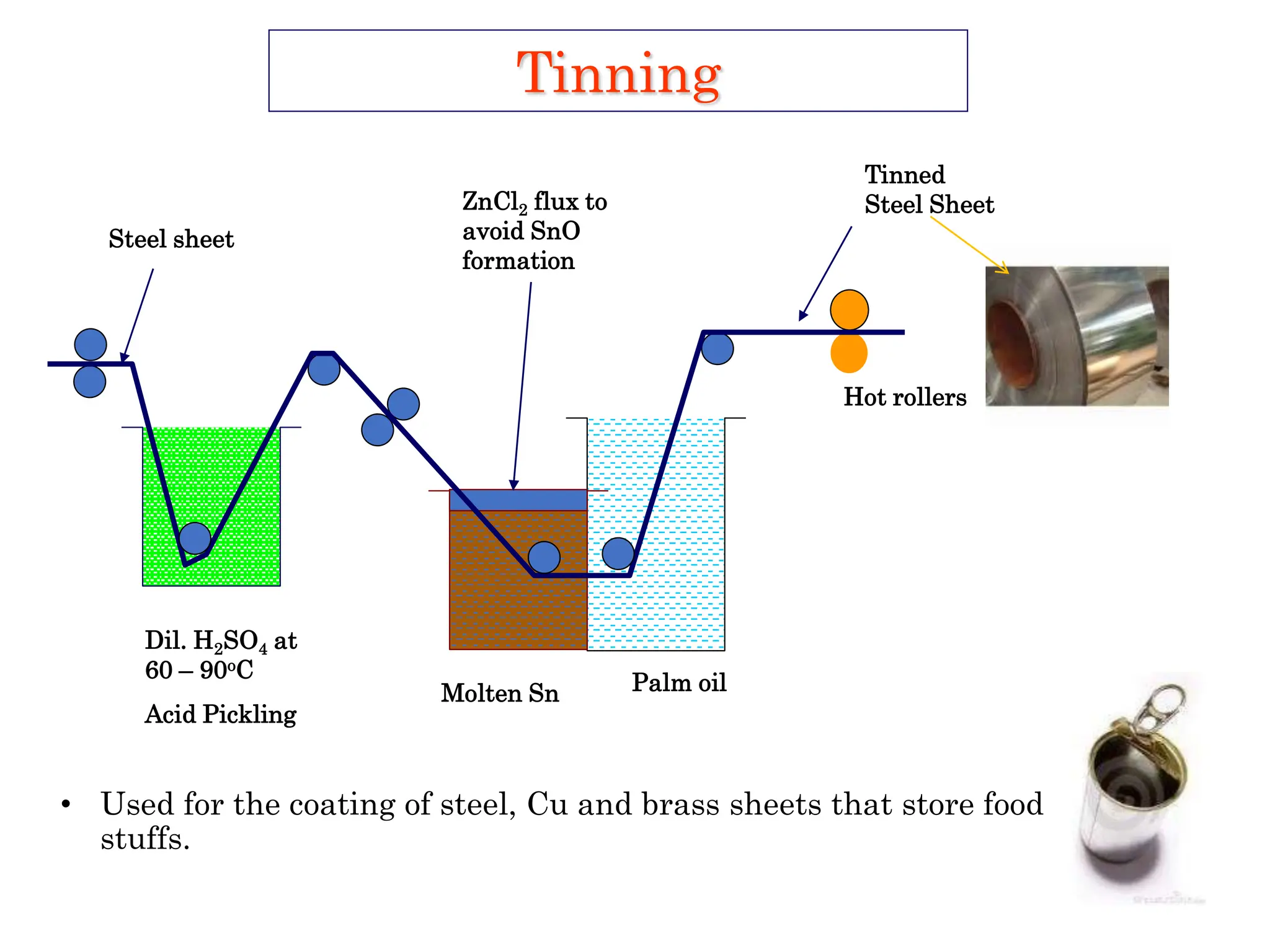
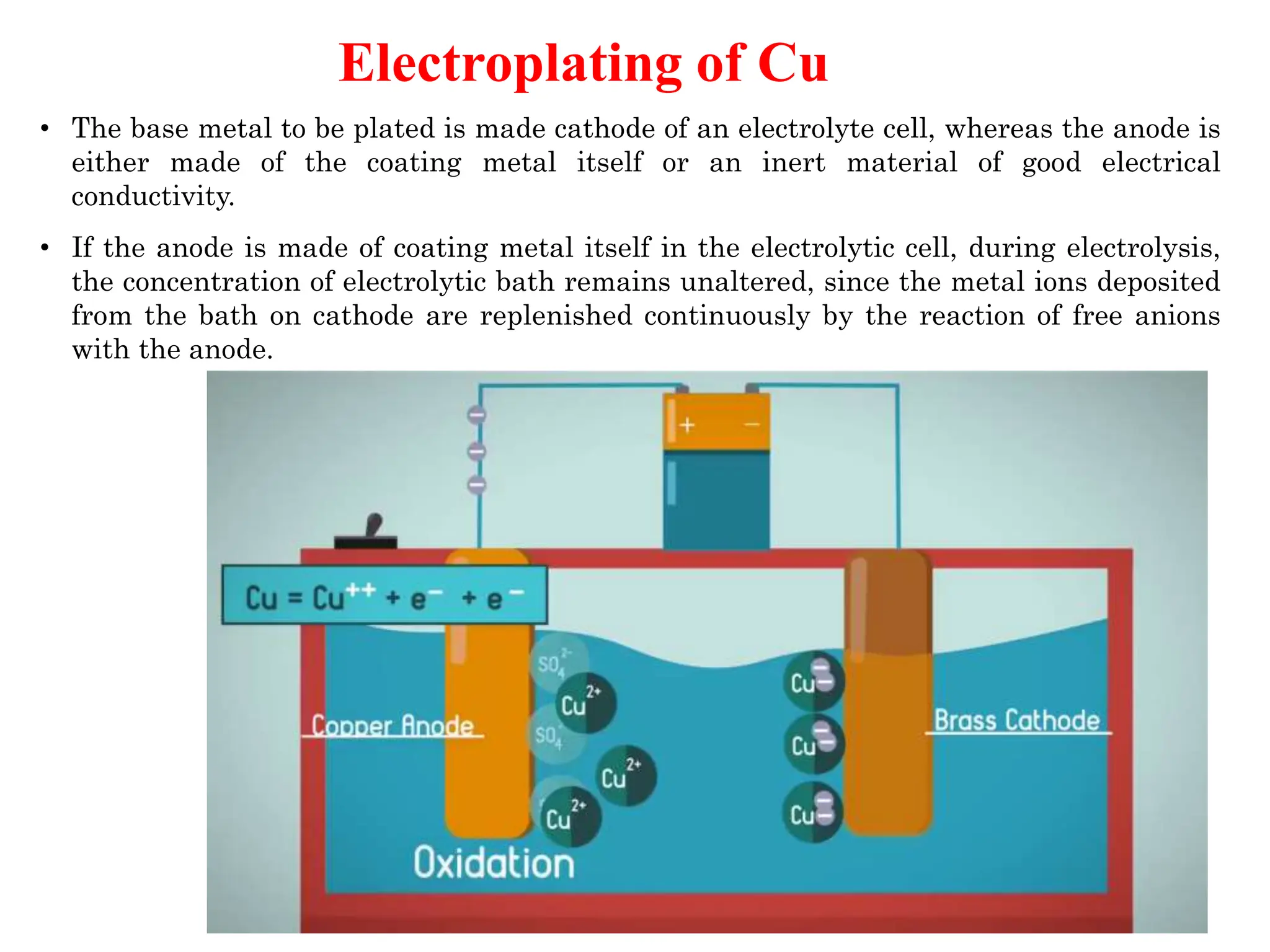
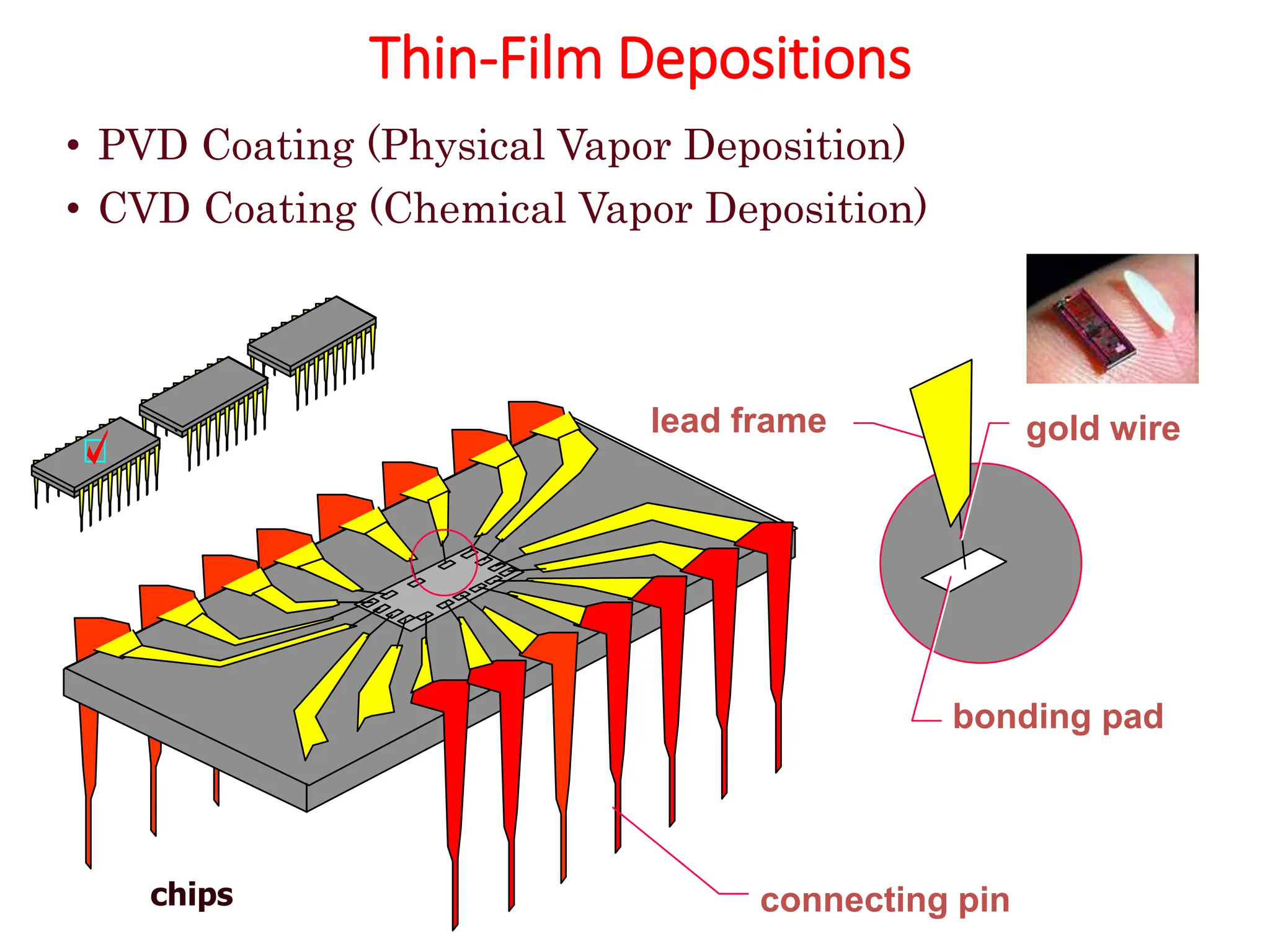
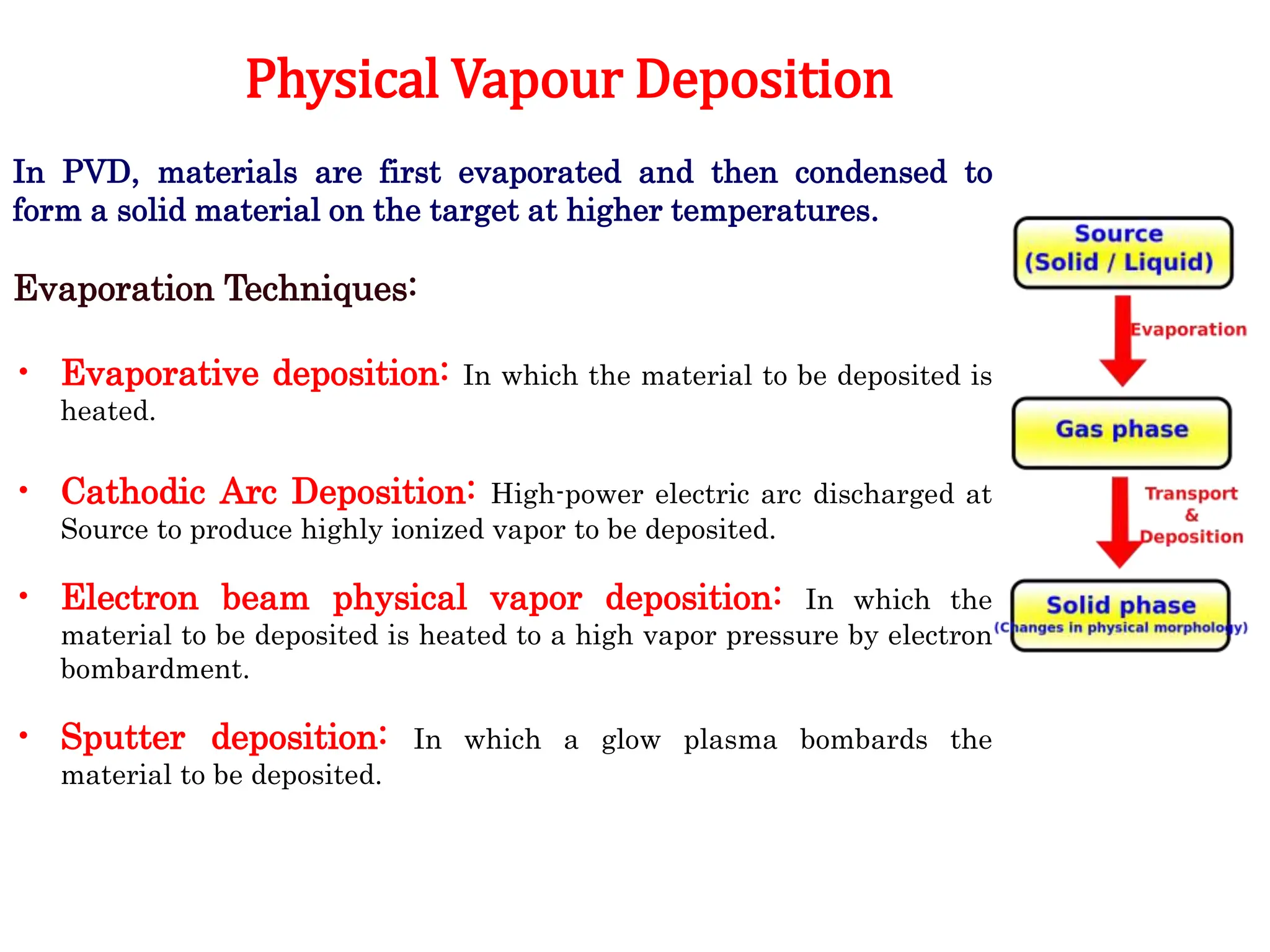
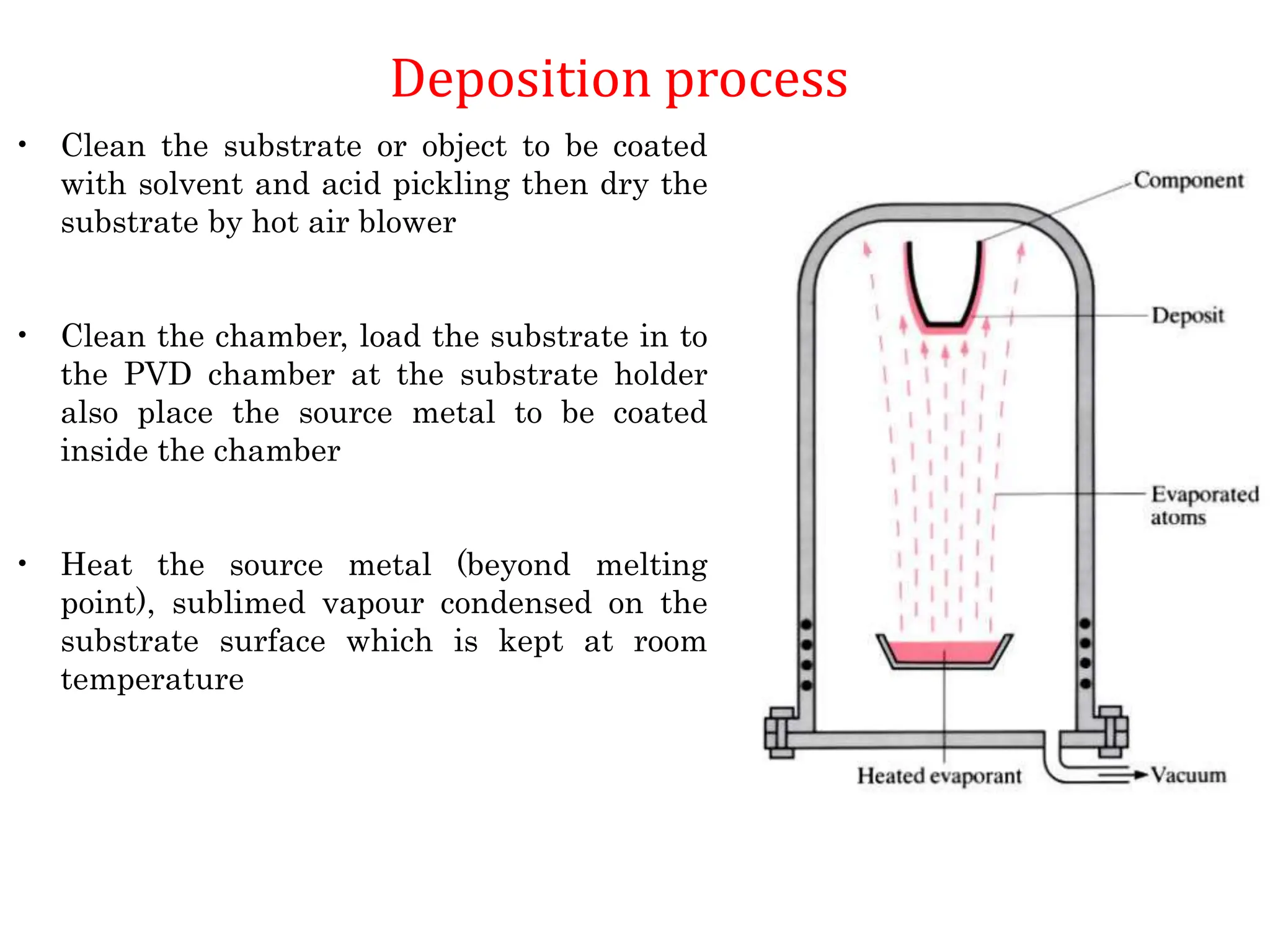
The document discusses corrosion, detailing its mechanisms, types (chemical and electrochemical), and factors affecting rates. It explains how metals deteriorate due to reactions with their environment, factors influencing corrosion, and methods for its control, including material selection and protective techniques like cathodic protection. Additionally, it highlights specific corrosion types like pitting and galvanic corrosion, alongside strategies for minimizing damage through proper design and inhibitors.