Corrosion engineering
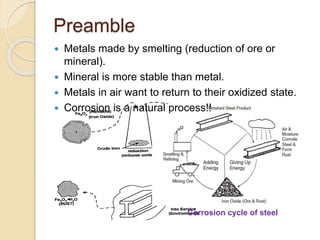
- 1. Preamble Corrosion cycle of steel Metals made by smelting (reduction of ore or mineral). Mineral is more stable than metal. Metals in air want to return to their oxidized state. Corrosion is a natural process!!
- 2. Definition Corrosion is defined as degradation or destruction of metal or an alloy because of chemical or electrochemical reaction with the surrounding environment or medium. Rusting is the corrosion related to iron and iron-based alloys. Non- ferrous metals corrode but do not rust.
- 3. Four requirements of corrosion Anode Cathode Current flow Electronic path } Cell
- 4. Reasons for corrosion studies Economic (due to material losses). Safety (to prevent catastrophic consequences resulting from operation failure of equipment). Conservation (to conserve metal resources, which are limited).
- 5. Responsibility for corrosion failure Wrong specification, 16 Bad inspection, 10 Human error, 12 Lack of proving, 36 Poor planning, 14 Unforseeable, 8 Other causes, 4
- 6. Bhopal disaster (1984) (Methyle iso cyanate) 4,000 dead 500,000 affected
- 7. Accident description As part of routine procedures, the pipes leading from the MIC distillation column to the storage tanks were regularly flushed with pressurized water. MIC and any associated products can be quite corrosive and could form corrosion deposits in the pipe. These deposits would contaminate the MIC in the tanks and could initiate unwanted reactions. During cleaning, valves in the product lines were to be closed and a blank or slipblind placed in the product line leading to the storage tank to prevent contamination. However the valves, although closed, were not sealing properly because of corrosion and the maintenance crew forgot about the blank. It appears that about 1000 kg of water plus metal debris entered into the tank and initiated an exothermic reaction.
- 8. Safety features of MIC tank Operative: ◦ Usual practice: Tank should be filled upto 50%. ◦ Prime protection: External jacketed cooling system (0ºC). ◦ Safety valve. ◦ Located under ground. Inoperative: ◦ Refrigeration system was turned off 6 months ago due to economic crisis of the company. ◦ Valves were defective due to lack of maintenance. ◦ Tank filled with more than 50%.
- 9. MIC tank
- 10. Economic factors Direct loss Replacing corroded structure and equipment Adding corrosion inhibitors Cost for corrosion- resistant metals Indirect loss Shutdown Loss of product Contamination of product Loss of efficiency Overdesign
- 11. Some examples rusted chilled water piping penetration at deck, due to water wicking under insulation that is flush to deck rusted steam piping under insulation at a fuel oil heater
- 12. Some examples (contd…) steel deck support brackets for topside vertical ladder (Naval ship) topside rusted steel electrical conduit clamps
- 13. Some examples (contd…) pipe hanger (Naval ship) floodlight positioning bracket (Naval ship)
- 15. Examples (contd…) Bicycle rim 15 Aluminum rim Chromium plated brass spoke Al - 1.66 volt Cr - 0.74 volt
- 16. Some examples (contd…) Railing of a bridge (Karatsu, Saga, Japan 6 May 2014)
- 17. Some examples (contd…) Cast iron pump impeller
- 18. Household examples (conted…) Mail box corrosion Plumbing fixtures corrosion
- 19. Pemex Refinery explosion Mexico (19 Sept 2012) 19 State-owned petroleum company Process crude oil to produce petrol, diesel, kerosene etc. Explosion occurred 26 died, 40+ injured Financial loss: $300 million – $1000 million
- 20. Guadalajara Sewer Explosion Mexico (1992) 20 Gasoline pipeline (Steel) was underneath the water pipeline (Zn coated iron) Corrosion occurred in both pipelines Gasoline came out and entered in a nearby sewer line 252 died; 500 injured; 15000 homeless
- 21. Consequences of corrosion Waste of metals ◦ 25% of annual world production of iron is wasted due to corrosion Decrease in efficiency of machineries Failure of machineries Leakage in the process ◦ Health & fire hazard Causes contamination 21
- 22. Who will study corrosion??? Distribution of disciplines Chemical Engineering Chemistry Civil engineering Electrical engineering None Materials engineering Business Physics
- 23. Expectation from you … Ensuring maximum life of new equipment through corrosion protection. Preservation of existing equipment. Improving the quality of product. Prevention of spillage or leakage. Reducing hazards to life and property.
- 24. Theories of corrosion Chemical or Dry corrosion Electrochemical corrosion or Wet corrosion
- 25. Chemical or Dry corrosion Simplest case of corrosion. Corrosion takes place due to direct chemical attack. Oxygen, halogens, hydrogen sulphide, nitrogen etc. Corrosion product may be insoluble, soluble or liquid product.
- 26. Classification Oxidation corrosion • Takes place by direct action of O2 • Absence of moisture Corrosion by other gases • CO2, SO2, Cl2, H2S, F2 • Extent of corrosion varies Liquid metal corrosion • Flowing liq at high temp. Chemical or Dry corrosion
- 27. Electrochemical or Wet corrosion There must be an anode & a cathode. There must be an electrical potential difference between the electrode. There must be a metallic path electrically connected with both electrodes. There must be an electrically conductive medium.
- 28. Difference Chemical corrosion 1. Takes place in dry condition. 2. Takes place by direct chemical attack. 3. Can take place on heterogeneous or homogeneous metal surface. 4. Uniform corrosion. 5. Corrosion product accumulates at the spot. Electrochemical corr. 1. Takes place in presence on wet condition. 2. Takes place through the formation of cell. 3. Can take place only on heterogeneous metal surface. 4. Non-uniform corrosion. 5. Corrosion product accumulates at the cathode.
- 29. Mechanism (general) Carbon electrode & zinc cup Reduction occurs at carbon electrode while oxidation occurs at zinc cup Zn0→Zn2+ + 2e- Amnt. of Zn corrosion W = kIt Corrosion occurs at zinc cup
- 30. Mechanism of corrosion (iron) 30 2(Fe → Fe2+ + 2e-) O2 + 2H2O + 4e- → 4OH - 2Fe + O2 + 2H2O → 2Fe(OH)2 2Fe(OH)2 + ½O2 + 2H2O → 2Fe(OH)3 { } Redish-brown
- 31. Local-action current & local- action cell Observed at metal surface while exposed in solution (water, salt solution, acids, or alkalies). Accompanied by chemical conversion of the metal to corrosion products. This happens due to impurities of a metal constitute the electrodes.
- 32. Types of cells Dissimilar electrode cells (e.g. dry cell) Salt concentration cells Differential aeration cells Differential temperature cells While connected, Cu dissolves at the anode and deposited at the cathode. Tending the CuSO4 solution to reach the same concentration.
- 33. Types of cells Same electrode material Same electrolyte Only difference is O2 concentration (causes potential difference) Example: crevice corrosion at the lamp post.
- 34. Differential temperature cell Same electrode material. Same electrolyte. Temperature difference in electrodes. Example: corrosion inside heat exchangers, boilers.
- 35. Forms of Corrosion General ◦ Identified by uniform formation of corrosion product Localized ◦ Caused by different chemical or physical conditions Bacterial ◦ Caused by formation of bacteria that has affinity to metal Galvanic / Dissimilar metal ◦ Caused when dissimilar metals come to contact 35
- 36. Corrosion damages Uniform corrosion Pitting corrosion Crevice corrosion Galvanic corrosion Intergranular corrosion Stress corrosion cracking (SCC) Based on the appearance of corrosion damage: 36
- 37. Corrosion damages Uniform attack Pitting ◦ Impingement attack ◦ Fretting corrosion ◦ Cavitation-erosion Dezincification and parting Intergranular corrosion cracking
- 38. Corrosion rate expression mm/y- millimeter penetration per year gmd- grams per square meter per day ipy- inches-penetration per year mpy- mils-penetration per year (1 mil = 0.001 in) Corrosion rate < 0.005 ipy (good corrosion resistance). 0.005 < Corrosion rate < 0.05 ipy (satisfactory). Corrosion rate > 0.05 ipy (unsatisfactory).
- 39. Free energy change (∆G) Chemical reaction mechanism ◦ More (-)ve ∆G, greater tendency of reaction to occur. kCalGOHMgOOHMg 6.142)( 2 1 0 222 kCalGOHCuOOHCu 6.28)( 2 1 0 222 kCalGOHAuOOHAu 7.15)( 4 3 2 3 0 222 Electrochemical reaction mechanism ◦ ∆G = - EnF ◦ Higher the value of E, greater tendency of reaction to occur.
- 40. Nernst equation Nernst equation provides an exact emf of a cell in terms of activities of products and reactants. .............. rRqQmMlL m M l L r R q Q aa aa nF RT EE ln0 Homework: Derive this equation. General reaction for Galvanic cell:
- 41. EMF series Metals arranged according to standard potential values. More positive → noble metals More negative → active metals Only useful to predict which metal is anodic to other. Valid when activity of metal ions in equilibrium are unity i.e. 1. Alloys are not included (Only pure metals are considered).
- 42. Electromotive force series Noble metals Active metals
- 43. Galvanic series Arrangements of both metals and alloys. Well representative of particular environment. More appropriate for practical situation.
- 44. Pourbaix diagram Represents thermodynamic state (thermodynamic data: Potential vs pH) Represents chemical & electrochemical equilibria between metal and aqueous solution and relates corrosion. Does not give any data on rate of reaction.
- 45. Pourbaix diagram for iron Horizontal lines represent reaction, which does not involve H+ or OH-. Vertical lines involve H+ or OH- but no electrons.Fe → Fe2+ + 2e- ; activity ≈ 10-6 Sloping lines involve H+ or OH- and electron. Fe2O3 + 6H+ + 2e- → 2Fe2+ + 3H2O
- 46. POLARIZATION What is polarization? Linkage between polarization and corrosion. Types of polarization. Corrosion control through polarization.
- 47. What is polarization? Electrodes are no longer in equilibrium when a net current flows. In a Galvanic cell: ◦ Anode potential moves towards cathode. ◦ Cathode potential moves towards anode. ◦ Thus the difference in potential becomes smaller. So the extent of potential change caused by net current flow to or from an electrode is called polarization.
- 48. Cu Zn CuSO4 ZnSO4 A V R log current Potential φCu φZn Imax Imax∙Re I∙(Re+ Rm) φcorr Polarization curves can never intersect.
- 49. Types of polarization Concentration polarization Activation polarization Polarization due to IR drop
- 50. Concentration polarization φCu = 0.342 volt φ1 = Potential of Cu electrode before current passing 1 2 1 log 2 0592.0 342.0 Cu When current flows, Cu2+ + 2e- → Cu0 2 2 2 log 2 0592.0 342.0 Cu 2 2 1 2 12 log 2 0592.0 Cu Cu
- 51. Significance of Concentration polarization Larger current flow causes smaller Cu ion concentration (Cu2+)2, which results larger polarization When (Cu2+)2, → 0 then (φ2 – φ1)→∞ The current density at this situation is called limiting current density. 2 2 1 2 12 log 2 0592.0 Cu Cu
- 52. Activation polarization Causes by slow electrode reaction Requires activation energy Example: reduction of hydrogen ion 2H+ + e- → H2
- 53. Influence of polarization Anodically controlled polarization Cathodically controlled polarization Resistance control
- 54. Anodically controlled Polarization occurs mostly at anode. log current Potential φC φA Imax φcorr Icorr φcorr Example: Impure lead surface immersed in sulfuric acid. Lead sulfate film will be formed and Cu (the impurity) will be exposed for corrosion.
- 55. Cathodically controlled Polarization occurs mostly at cathode. log current Potential φC φA Imax φcorr Icorr φcorr Examples: Zn corrodes in sulfuric acid. Iron corrodes in water.
- 56. Resistance control Electrolyte resistance is very high. Resultant current is not sufficient to polarize either anode or cathode. log current Potential φC φA Icorr Examples: Porous coating covering a metal surface. R∙Icorr
- 57. Principle of cathodic protection Polarization of cathode is done by supplying external current Electrochemical potential of cathode moves in negative direction (towards anode) Auxiliary anode is used to spread current The material is protected when it reaches protection potential 57
- 58. Types of cathodic protection CP with sacrificial anode CP with impressed current 58 Fe → - 0.44 v (noble) Mg → - 2.37 v (active)
- 60. Passivity Fe in concentrated HNO3 → No reaction (Passive state) Fe in dil. HNO3 → Rapid corrosion reaction (Active state) Passivity is the phenomenon that demonstrate how the corrosion is inhibited in any given environment. 70% concentrated HNO3 Fe Dilute
- 61. Characteristics of Active-Passive metal The same metal can act as active as well as passive depending on the situation. Passivity occurs because a film is produced on the metal surface. Thickness of film ≤ 30Å. (1Å = 1×10-7 mm)
- 62. Potentiostatic polarization curve of active-passive metal (Fe) Active state: metal corrodes (Fe0 → Fe2+ + 2e-) Passive state: insulative film is formed & no corrosion occurs Transpassive zone: Formation of Fe3+ as well as O2 evolution log i Potential(φ) passive icritical ipassive P
- 63. Flade potential of Fe When applied potential is withdrawn, passivity decays Passivity decays in a very short time At Flade potential, active state of the metal is re- established. Time (sec) Potential(φ) φF Important P and φ are roughly equal (but not same). WHY? ◦ change in pH ◦ IR drop due to insulating film
- 64. Passivators of iron Passivators are inorganic oxidizing agents, which reacts slowly when in direct contact with iron. They are adsorbed on the metal surface. Higher the concentration of passivator, more readily it adsorbs CrO4 2-, NO2 -, MoO4 2-, WO4 2-, FeO4 2-
- 65. Theory of passivity Oxide film theory ◦ Metal oxide or other compound is formed ◦ This oxide separates metal from the environment ◦ Eventually slows down the rate of reaction ◦ Effectiveness of corrosion reduction depends on the nature & properties of thin protective film.
- 66. Theory of passivity Adsorption theory ◦ Passivity is achieved due to chemisorbed film of O2 or other passivating agents ◦ This film separates metal from water or other corroding environment ◦ Film may be of monolayer or multilayer H Hmonolayer Thick layer (multilayer) H Oxygen Metal Hydrogen
- 67. Passivity in iron alloys Fe alone is not naturally passive (i.e. corrodes in short time) Cr is a naturally passive metal (i.e. remains bright & tarnish-free) Fe-alloy have passive property when at least 12% Cr is there CorrosionRate 2 4 14 180 Chromium (wt%) 6 8 10 12 16 20
- 69. Effect of oxygen on MS corrosion Critical concentration may change: ◦ Increases with increasing T ◦ Decreases with increase in velocity Concentration of dissolved O2 (ml/L) Corrosionrate(gmd) Critical concentration
- 70. Effect of temperature Temperature (°C) Corrosionrate(ipy) Open system Corrosion rate increases with increase in T In open system: Rate increases first Then falls down at 100°C In closed system: O2 can not escape Rate increases with T, until all O2 is consumed 100°C Such falling off is related to decrease of O2 solubility in water as T is raised.
- 71. Effect of pH on iron corrosion pH >10 Higher surface pH Because of alkali & dissolved O2 iron gets passivated CorrosionRate(ipy) 12 10 8 6 4 214 pH
- 72. Effect of pH pH 4 ~10 Corrosion rate is independent of pH Rate depends on O2 diffusion to the iron surface Diffusion barrier (FeO) is regenerated Surface pH always remains at 9.5 throughout this range (Why??) CorrosionRate(ipy) 12 10 8 6 4 214 pH
- 73. Effect of pH pH < 4 FeO film dissolved Surface pH decreases Corrosion increases (because of H2 evolution & O2 development) CorrosionRate(ipy) 12 10 8 6 4 214 pH
- 74. Effect of velocity (Freshwater) Corrosion increases with velocity because O2 contact with the surface At sufficient high velocity, enough O2 reach at the surface, which causes partial passivation At further increase in velocity, corrosion-product film is eroded CorrosionRate(ipy) 2 4 6 80 V (ft/s) Rough steel Polished steel
- 75. Effect of velocity (seawater) Corrosion increases with velocity Passivity is never achieved CorrosionRate 2 4 6 80 V (ft/s) High concentration of Cl-
- 76. Corrosion damages Uniform corrosion Pitting corrosion ◦ Impingement attack ◦ Fretting corrosion ◦ Cavitation-erosion Crevice corrosion Galvanic corrosion Intergranular corrosion Stress corrosion cracking (SCC) Based on the appearance of corrosion damage:
- 77. Uniform corrosion Results from uniform penetration over the surface Also results from multiple local-action cell Location of anodic & cathodic areas move on the surface Examples: atmospheric exposure of metal (rusting of steel, green patina formation of copper), exposure in salt water or soil or chemicals Rusting of steel highway bridge
- 78. Prevention of uniform corrosion Proper material selection Use of coating or inhibitor Cathodic or anodic protection Individual or combination of all the above
- 79. Pitting corrosion Highly localized form of corrosion Causes from local inhomogeneneity on metal surface, local loss of passivity, rupture of protective oxide coating. Produce sharp holes (small or large in diameter) Examples: iron buried in soil (shallow pits), carbon steel in contact with HCl (deep pits), SS immersed in seawater.
- 80. Pitting factor Pitting factor = 1 (uniform attack) d p Pitting factor = Deepest metal penetration Average metal penetration = p d
- 81. Mechanism of Pitting Example: Metal in NaCl solution
- 82. Mechanism of Pitting Example: Metal in NaCl solution M+ is pitted by aerated NaCl solution Once a pit is created, local environment & surface film become unstable Rapid dissolution occurs within the pit while O2 reduction takes place on the adjacent surface (self propagating process) Rapid dissolution of M+ causes excess +(ve) charge in the pit, which causes migration of Cl- in the pit. High concentration of metal chlorides (M+Cl-) & hydrogen ion in the pit. H+ and Cl- stimulate dissolution of metals and alloys.
- 83. Impingement attack Moving liquid particles cause the damage. Metals subject to high-velocity liquid. Corrosion-erosion is another name. Example: Copper and brass condenser tubes.
- 84. Fretting corrosion Combination of corrosion and wear Oxidation is the most common element Relative movement between two surfaces Metal oxides become trapped between two surfaces and causes wear Examples: rolling contact bearing Prevention: Lubrication Restricting the degree of movement
- 85. Cavitation-erosion Cavitation ◦ Repetitive low & high pressure areas developed ◦ Consequently bubbles form & collapse at metal-liquid surface Damage caused by cavitation is called cavitation damage Metal surface becomes pitted Examples: blade/rotor of pumps, water turbine blades
- 86. Prevention of Pitting Lessen the aggressiveness of the environment (e.g. Cl- concentration, temperature, acidity etc.) Upgrade materials of construction (e.g. Cr (12%) containing SS, Mo (4-6%) containing SS etc.) Modify the design of system (e.g. ensure proper drainage, avoid crevices etc.)
- 87. Galvanic corrosion Metal or alloy electrically coupled with another metal or conducting nonmetal The system should have common electrolyte Materials possessing different surface potential Driving force ->>>> potential difference between two dissimilar metal Aluminium rim and chromium plated brass spoke. Mud on the rim acts as electrolyte.
- 88. Galvanic and electrolytic cell In Galvanic cell reactions occur spontaneously when connected by electrolyte. Chemical energy is converted to electrical energy. Examples: AA batteries, car battery (when it is being discharged). In electrolytic cell reactions do not occur without applying an external potential. Electrical energy is used to cause the desired chemical reaction. Examples: electroplating of Cu, Au, Ag etc., Car battery (when it is being charged).
- 89. Area concept of corrosion Corrosion of the anode may be 100 ~ 1,000 times greater than if the two areas were the same. What to do!!!!!!! Fe => φ = - 0.403 volt Cu => φ = + 0.521 volt Rivet = Fe Plate = Cu (i) Rivet = Cu Plate = Fe (ii) Corrosion of (i) >> corrosion of (ii)
- 90. Aloha aircraft incident 1 fatality and 7 injured. Why this occurred?? Corrosion occurred in lap joint. Corrosion product was Al(OH)3. Al(OH)3 expanded inside the lap joint and lead to pillowing. This created undesired increased level of stress. This stress produced cracking.
- 91. Prevention of Galvanic corrosion Avoid combinations in which the area of the less noble material is relatively small. Insulate dissimilar metals if possible. Apply coating e.g. teflon coating. Use chemical inhibitors, which reduces corrosiveness of the environment.
- 92. Inter-granular corrosion Localized type of attack at the grain boundary of metal. Grain boundary (small in area) acts as anode. Rest of the grain (larger area) acts as cathode. Attack penetrates deeply into the metal. Causing catastrophic failure.
- 93. Stress corrosion cracking (SCC) Metal subject to constant tensile stress & exposed simultaneously to a corrosive environment. Thus metal suffers cracking called SCC. Compressive stress is not damaging. Example: Riveted steam boiler. High strength alumina alloy SCC
- 94. Riveted steam boiler Boiler water generally treated with alkali. Crevice between rivets & boiler plate allow alkali to concentrate. Concentration of alkali in crevices induce corrosion. Such type of corrosion is often called caustic embrittlement.
- 95. Remedy from SCC Severe cold working. Heat treatment (quenching or slow cooling). Cathodic protection. Use of special alloy (addition of Al, Ti etc.). Use of inhibitors (NaNO3 in boiler water, crude quebracho extract). CorrosionRate Carbon steel (0.076% C) 200 400 600 8000 Temp (°C) 1000 Zone refined steel (pure steel)
- 96. Atmospheric corrosion Atmosphere: 79% N2, 21% O2 (CO, CO2, NH3, H2S, SO2, NOx, suspended particles) Based on the pollutants: ◦ Rural atmosphere (little or no contaminants) ◦ Marine atmos. (high moisture & Cl-) ◦ Urban atmos. (NOx, CO, CO2) ◦ Industrial atmos. (CO, CO2, SO2) One metal is resistive to a particular atmosphere but not effective in the other. Example: (i) Galvanized steel (C.I. sheet) in rural atmos. but less resistive in industrial atmos. (ii) Lead performs better in industrial atmos. Because PbSO4 film is developed.
- 97. Corrosion film-product Metal surfaces retaining moisture corrode rapidly compared to those exposed fully. Why???? Because H2SO4 absorbed by rust accelerates corrosion. Painting just after rainy season is very efficient than painting in winter. 4232 2 1 342 4 1 4 2 1 2 3 2 1 2 1 2422242 SOHOFeSOFeFeSOFe OHSOHOOSOH
- 98. Atmospheric corrosion of steel Lossofweight(kg/m2) 2 4 6 80 Time (years) 10 Pure iron => powdery loose product (i.e. unstable film) Cu bearing low-alloy steel => compact rust film (i.e. stable)
- 99. Factor affecting atm. corrosion Dust content, gases in the atmos., moisture etc. Dust content: Suspended particle matters (SPM) e.g. carbon and carbon compound, metal oxides, NOx etc. SPM combines with moisture and produces Galvanic or differential aeration cell. Dust free air is less responsible for corrosion. In Dhaka: 3000 μg/m3 (allowed 400 μg/m3)
- 100. Factor affecting atm. corrosion Gases in atmosphere: H2S causes tarnishing of Ag, Cu, Ni. SO2 is most harmful S + O2 → SO2 2SO2 + O2 + 2H2O → H2SO4 Patina: Cu exposed to industrial atmosphere forms a greenish protective layer (CuSO4∙3Cu(OH)2). Fogging: Ni exposed to industrial atmosphere forms a tarnish of nickel sulfate. (But Ni is resistant to marine atmosphere).
- 101. Remedial measures of atmos. cor. Use of organic, inorganic or metallic coating. Reduction of relative humidity. Use of alloy. Slushing compounds (greases, oil, wax, organic additives etc.).
- 102. Underground corrosion Important because protection needed for thousands of kilometers of underground cross-country pipeline. NG, crude oil, water. Soil corrosion resembles atmospheric corrosion. Performance of any particular metal varies from one place to another over the country. ◦ Differences in pH ◦ Differences in soil composition ◦ Differences in moisture content
- 103. Factors affecting underground corr. Aeration of soil (depends on porosity). Electrical conductivity or resistivity. Dissolved salts (Na2SO4, NaCl are harmful). Moisture or water content (in desert, corrosion of buried metal is almost zero). pH (acidity or alkalinity).
- 104. Pitting characteristics of buried metal p = ktn (p: depth of the deepest pit, t: time, k & n: constant). ◦ n ≈ 0.1 for steel in well-aerated soil ◦ n ≈ 0.9 for steel in poorly-aerated soil Pits tend to occur more on the bottom side of the pipeline. ◦ Pipe settles down & air space produced on the top
- 105. Remedial measures of soil cor. Use of organic or inorganic coating (coal tar, pigments, portlant cement, vitreous enamel etc.). Metallic coating (Zn coating). Alteration of soil (layer of limestone chip surrounded the buried pipe). Cathodic protection (CP).
- 106. Corrosion prevention How to do this???? Change the metallic material. Altering the corrosive environment (pH, acidity, temp.). Separating the metal from environment (insulation). Providing appropriate design.
- 107. Other aspects of corrosion prevention Welding is preferable from riveting (crevice corrosion). Easy drainage and cleaning (design aspect). Avoid sharp bends (erosion-corrosion). Hot spots should be avoided (corrosion due to temperature gradient). Avoid electric contact (galvanic corrosion).
- 108. Factors influence the service life
- 109. Corrosion control by proper design Design for drainage (a) poor, (b) better (a) (b) Prevention of excessive turbulence Fluid trap between two metal jointsAvoid condensation droplets
- 110. Corrosion control by proper design Mixing vessel (a) poor, (b) better Prevention of localized cooling (a) poor, (b) better
- 111. Cathodic protection (CP) Basics of CP: External electric current is applied Cathodic potential is lowered to anodic direction Surface becomes equipotential Corrosion current no longer flows CP can not be used- Where??? In nonconducting liquids (oil) In extremely corrosive environment (theoretically possible but incurs huge cost) In electrically screened areas In vapor
- 112. CP with sacrificial anode Directly connect with a more active metal. Anode of this system is called sacrificial anode.
- 113. Application of CP Underground tanks Condenser water boxes Structures e.g. bridges Evaporators Valves, piping and other metal surfaces submerged in a liquids or constructed underground 113
- 114. CP with sacrificial anode Sacrificial anode is useful when electric power is not readily available. Low cost installation. Low maintenance cost. Combination with coating is better. Mg anode 8 km coated pipe 30 m bare pipe only
- 115. Overprotection Moderate overprotection is not harmful. Waste of electric current. Increased consumption of auxiliary anode. So much H2 may be produced, this may create H2 overvoltage (H2 embrittlement).
- 116. Alteration of environment Corrosion can be reduced by ◦ (i) changing the corrosive environment ◦ (ii) using inhibitors and passivators Moisture can be removed by dehumidification Dissolved O2 (by deaeration, saturation with N2, using O2 excavengers e.g. Na2SO3, N2H4). Cl- ions can be removed. Particulate solids can be removed.
- 117. Use of inhibitors Very specific to particular environment. Developed by empirical experiments. Sometimes proprietory in nature & composition is not disclosed. Usually used in closed or re-circulating system. Not used in once-through system.
- 118. Classification of inhibirots Passivators (inorganic oxidizing substances e.g. Na2CrO4, NaNO2, MoO4 2-). Organic inhibitors (Slushing compounds: wax, greases, oil). Vapor phase inhibitors (dicyclohexyl ammonium nitrite: nontoxic & odorless). 1 g of DAN saturates 550 m3 (20,000 ft3) of air.
- 119. Corrosion control through coatings Metallic coatings Inorganic coatings Organic coatings
- 120. Metallic coating How to do??? Hot dipping (specimen immersed in molten Zn or Steel bath). Electroplating (Nickel on brass). Metal spraying. Cementation (specimen put into metal powder at high temperature). Coating by gas phase reaction CrFeClFeCrCl 2 3 2 3 32 Coating by chemical reduction (electroless plating of Ni: Nickel phosphorus or Nickel-boron alloy coating). Ion implantation
- 121. Classification of metal coatings Noble coating (with Ni, Ag, Cu, Pb, Cr on steel). Sacrificial coating (Zn, Cd on steel). Noble coating
- 122. Metal cladding Cladding is a physical process in which a thin layer of one metal is brought in contact with a heavy layer of a base metal and binding by a combination of heat and pressure. Metal-to-metal laminar composite. Techniques: hot-roll bonding, cold-roll bonding, explosive bonding, weld cladding etc. Most engineering metals & alloys can be clad. Applied in pressure vessels, reactors, heat
- 123. Inorganic coating Vitreous enamel coating Powdered glass applied on metal surface and heated in furnace. Hard glassy external layer. Susceptible to mechanical damage or cracking by thermal shock. Portland cement coating Used to protect cast iron or steel on water or soil or both. Thickness is 5 to 25 mm. Low cost coating. Susceptible to mechanical damage and thermal shock. Chemical conversion coating Formed in situ by chemical reaction with metal surface. Anodic oxidation (anodizing) of metal (e.g. Al2O3). Phosphate coating on steel (Parkerizing /Bonderizing); (e.g.
- 124. Organic coating Includes paints, varnishes and lacquers. Paint: mixture of insoluble pigments (metal oxides; e.g. TiO2, Pb3O4, Fe2O3, ZnCrO4, PbCO3, BaSO4, clay etc.) in organic vehicle (natural oil). ◦ Paint is not useful to protect buried structures. ◦ Natural oil based paints not recommended for metal structures totally immersed in water. Varnishes: mixture of drying oil, dissolve resin and volatile thinner. Lacquers: resin dissolved in volatile thinner.
- 125. Filiform corrosion Self propagating crevice corrosion. Localized form of corrosion that occur under the coating or paint. Steel, aluminum and other alloys are affected. Particular concern in food packaging industry. “Wormlike” visual appearance. Occurred due to microenvironment effect.
- 126. Basics of a boiler operation Steam boiler consists of low carbon steel. Water inside the tube; hot gases around the tube. Generated steam passes through higher alloy steel. Dissolved O2 is removed (deaeration). 3Fe+4H2O→Fe3O4+4H2 (Inside the tube) At T>570°C: FeO formation. Cooling of steam: 4FeO→Fe3O4+Fe Magnetite: protective film (@570°C) Four-pass fire-tube boiler
- 127. Corrosion in boiler Protective magnetite layer may be damaged either chemically or mechanically. Pitting may occur in localized region. Excess OH- concentration (chemical damage). Differential concentration of oxide & metal (mechanical damage).
- 128. Boiler water treatment Why needed??? To control corrosion. To prevent scaling of boiler tubes (lowers heat transfer rate). To reduce SiO2 (damages turbine blades). Steps??? Removal of dissolved gases (O2, CO2). Addition of alkali. Use of inhibitors.
- 129. Removal of dissolved gases Dissolved gases causes pitting corrosion in tubes. Deaerated by steam Deaerated by O2 scavengers (Na2SO3, N2H4). Dissoved CO2 should be removed (carbonic acid is corrosive to steel). CO2 accumulation is avoided by CO2 release during boiler blowdown.
- 130. Alkali addition Alkali (NaOH) addition is usual practice. Caustic embrittlement may occur. NH3 is sometimes added instead of NaOH. ◦ NH3 is volatile. ◦ Does not accumulate in crevices. ◦ Crevice corrosion or SCC do not occur. HCl (ppm) NaOH (ppm) Relativeattack pH1 4 137
- 131. Corrosion testing What is it?? Is a powerful tool to control corrosion (flight). Is needed in design stage and in operational phase. Provides data useful for selection of materials: existing or alternative or new. Classification??? Laboratory testing Pilot-plant testing Field testing
- 132. Purpose of corrosion testing To evaluate and select materials. To obtain reference or database information. To determine quality-control and material acceptance requirement. To monitor corrosion-control programs. To identify research parameters and corrosion mechanisms. <Lack of service history, lack of time and budget.>
- 133. Testing, inspection, monitoring Operational data (On-line and off-line) Testing Inspection Monitoring Data Management Analyses Forecasting Decision making Engg. Review Assessment Maintenance
Editor's Notes
- Objective #2
