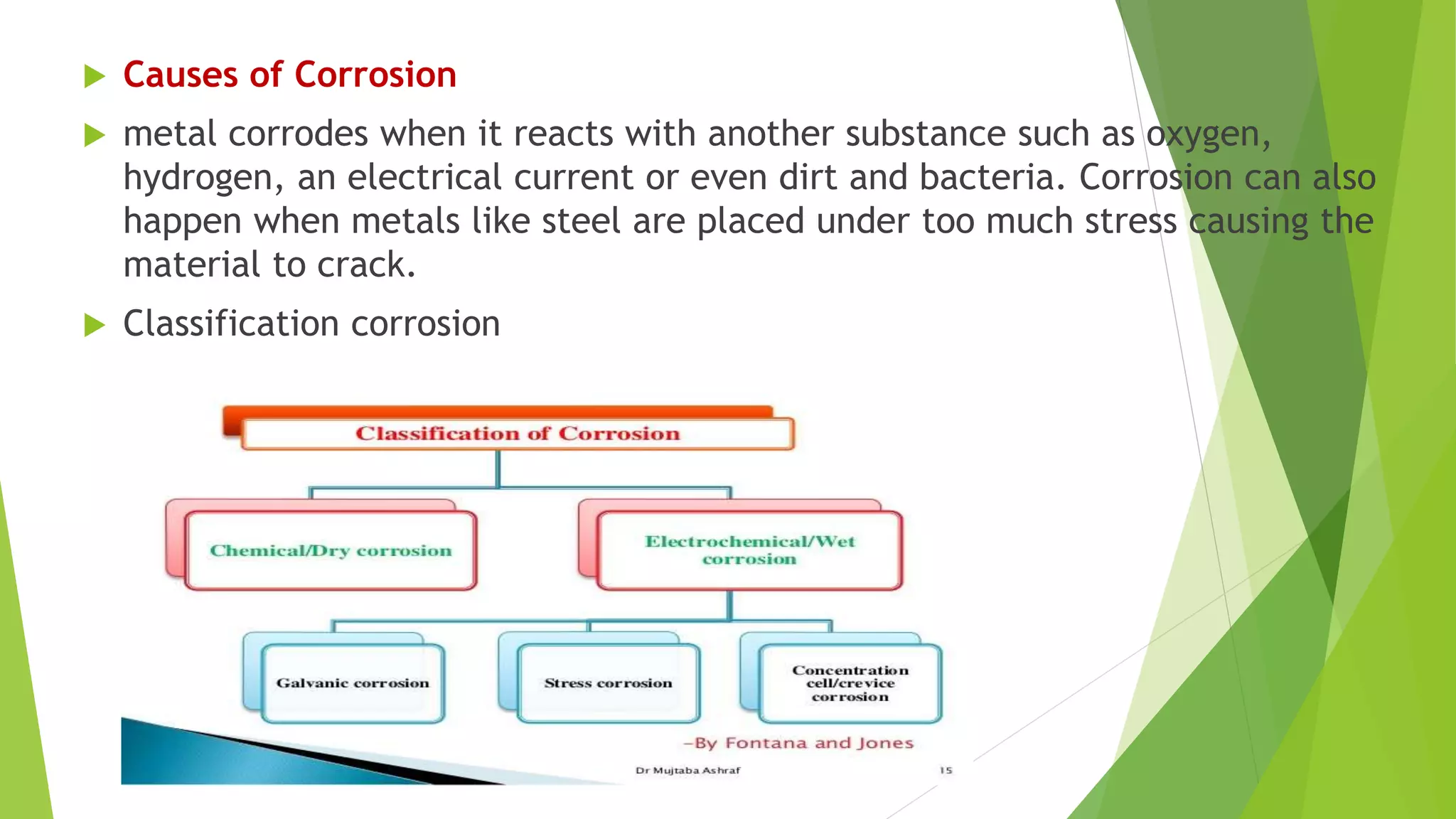
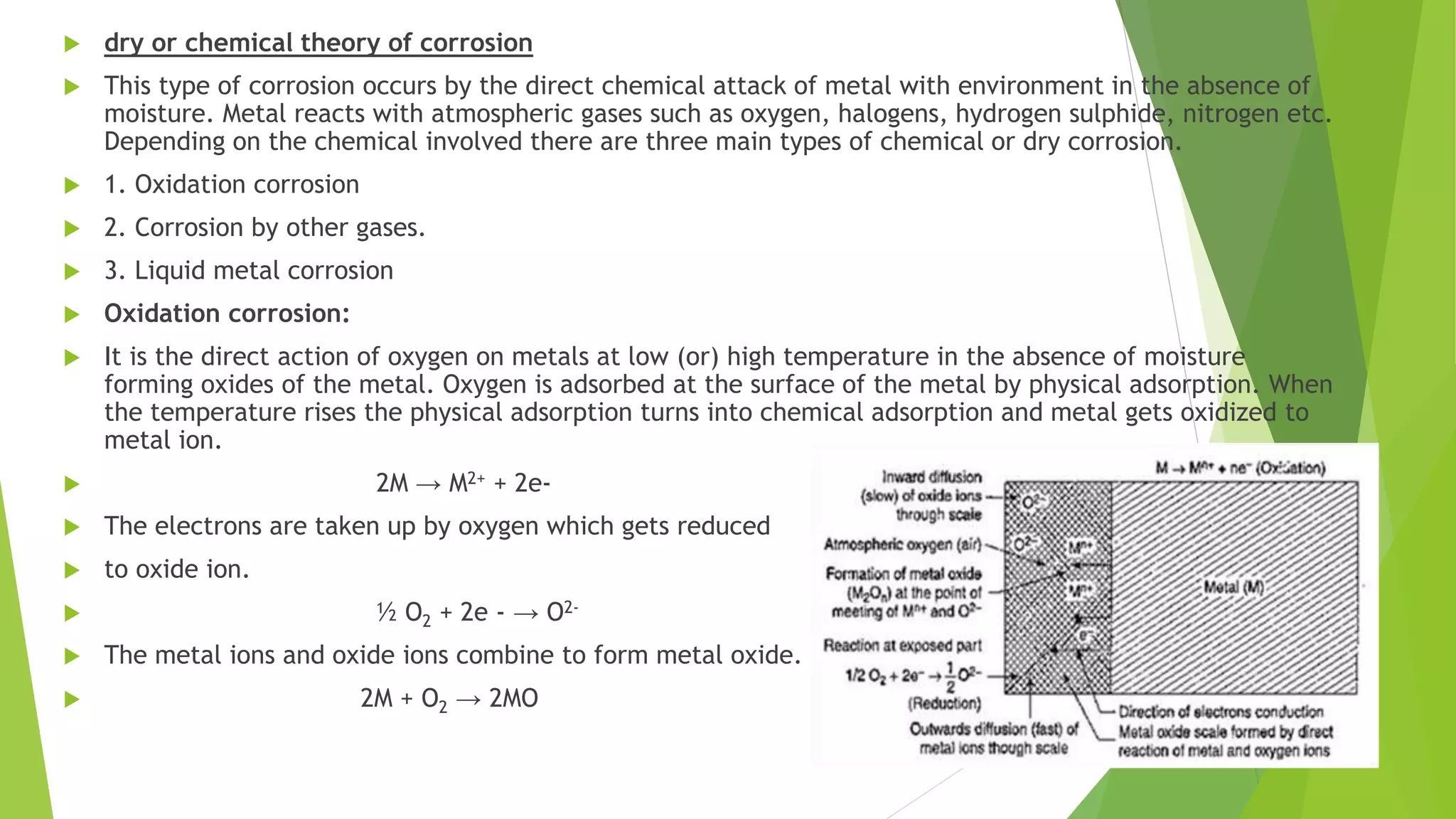
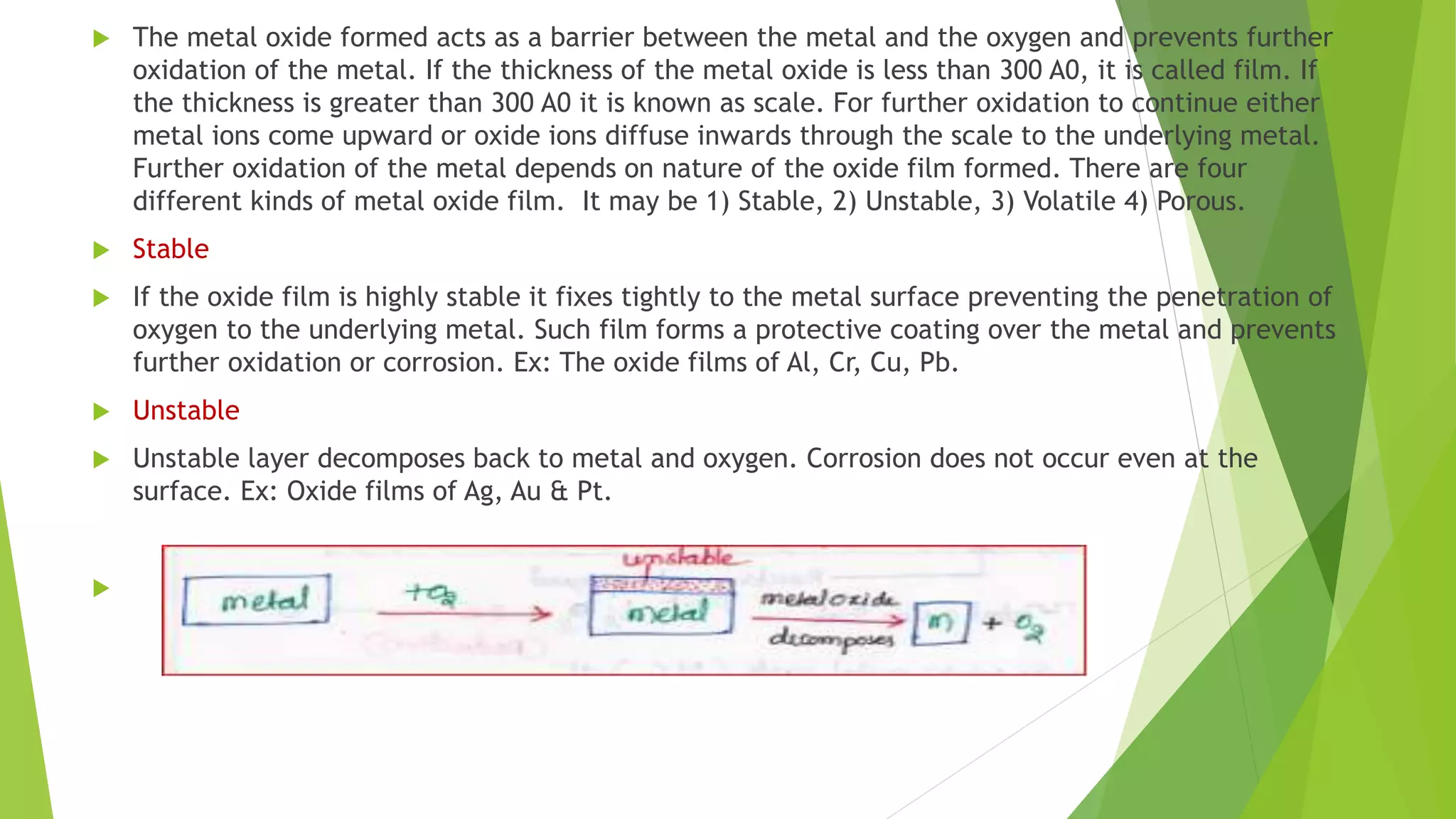
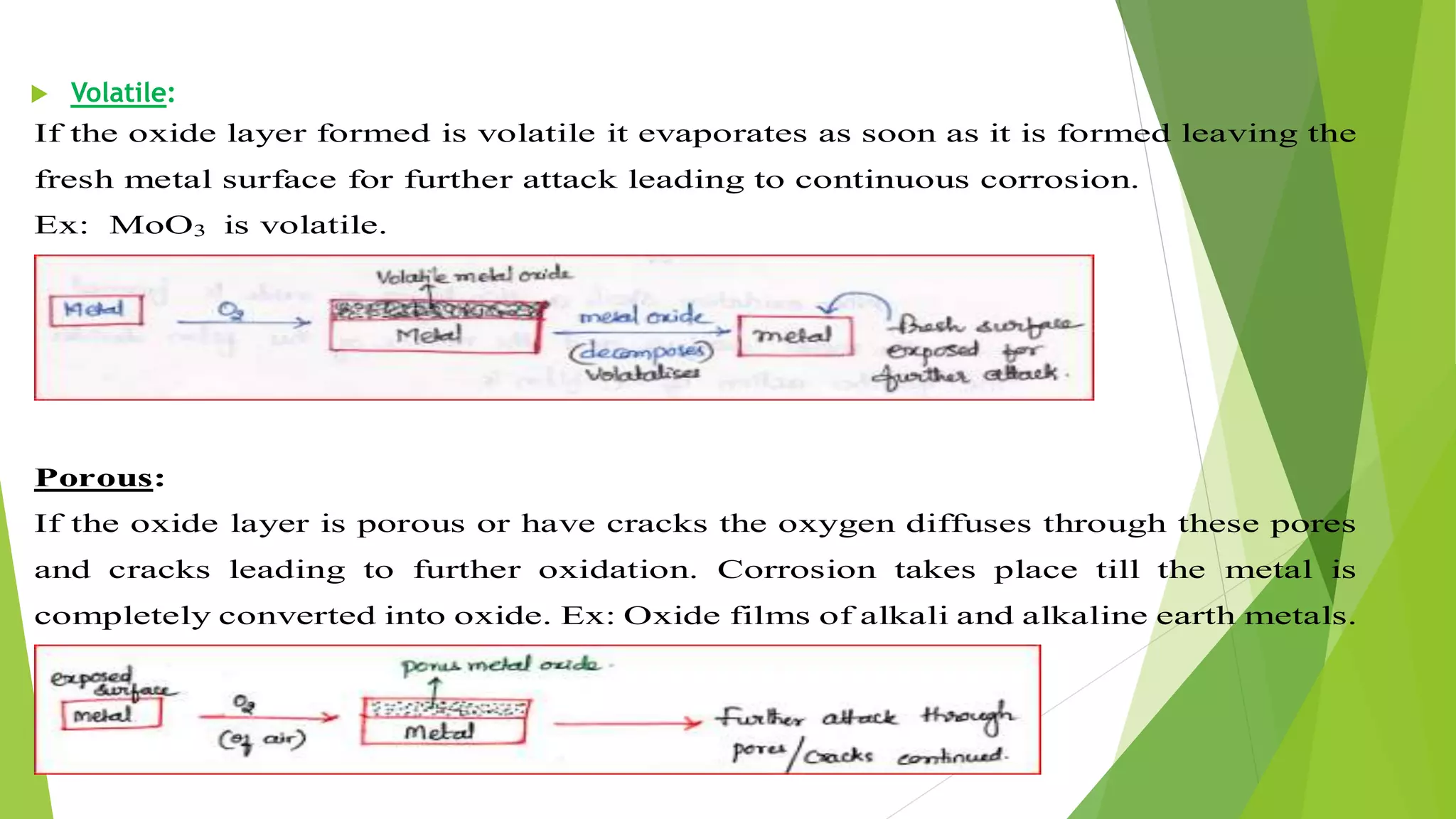
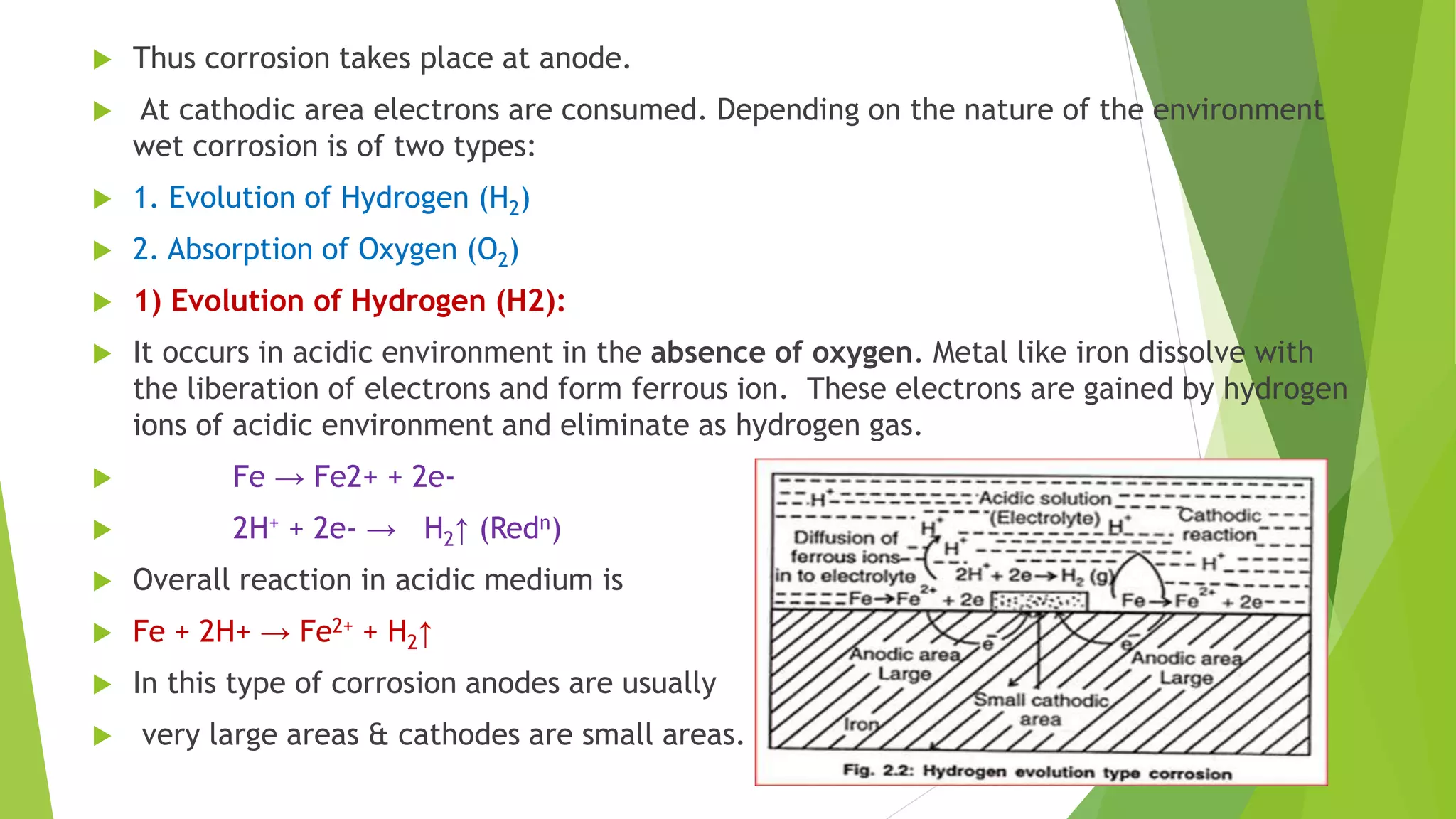
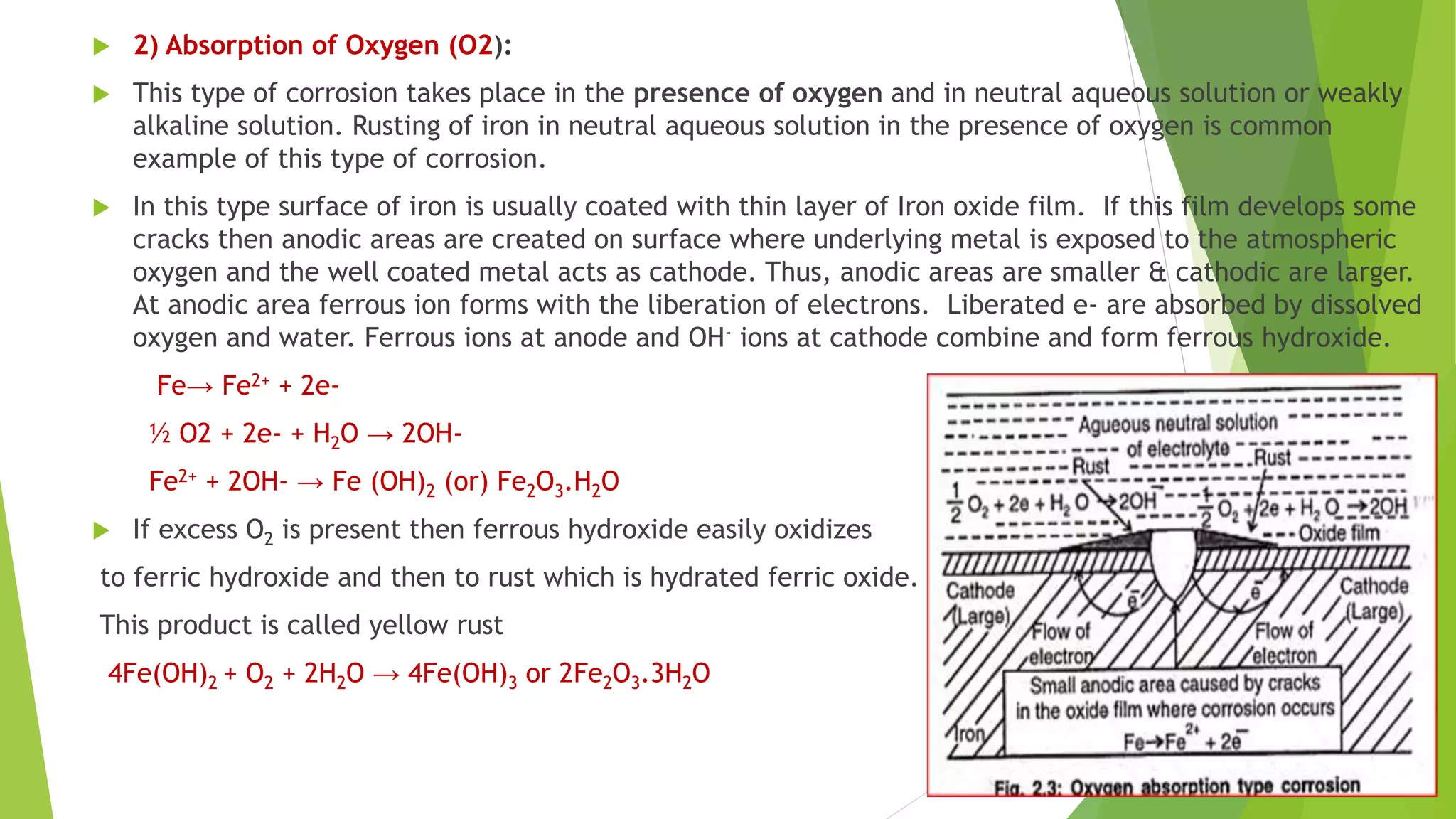
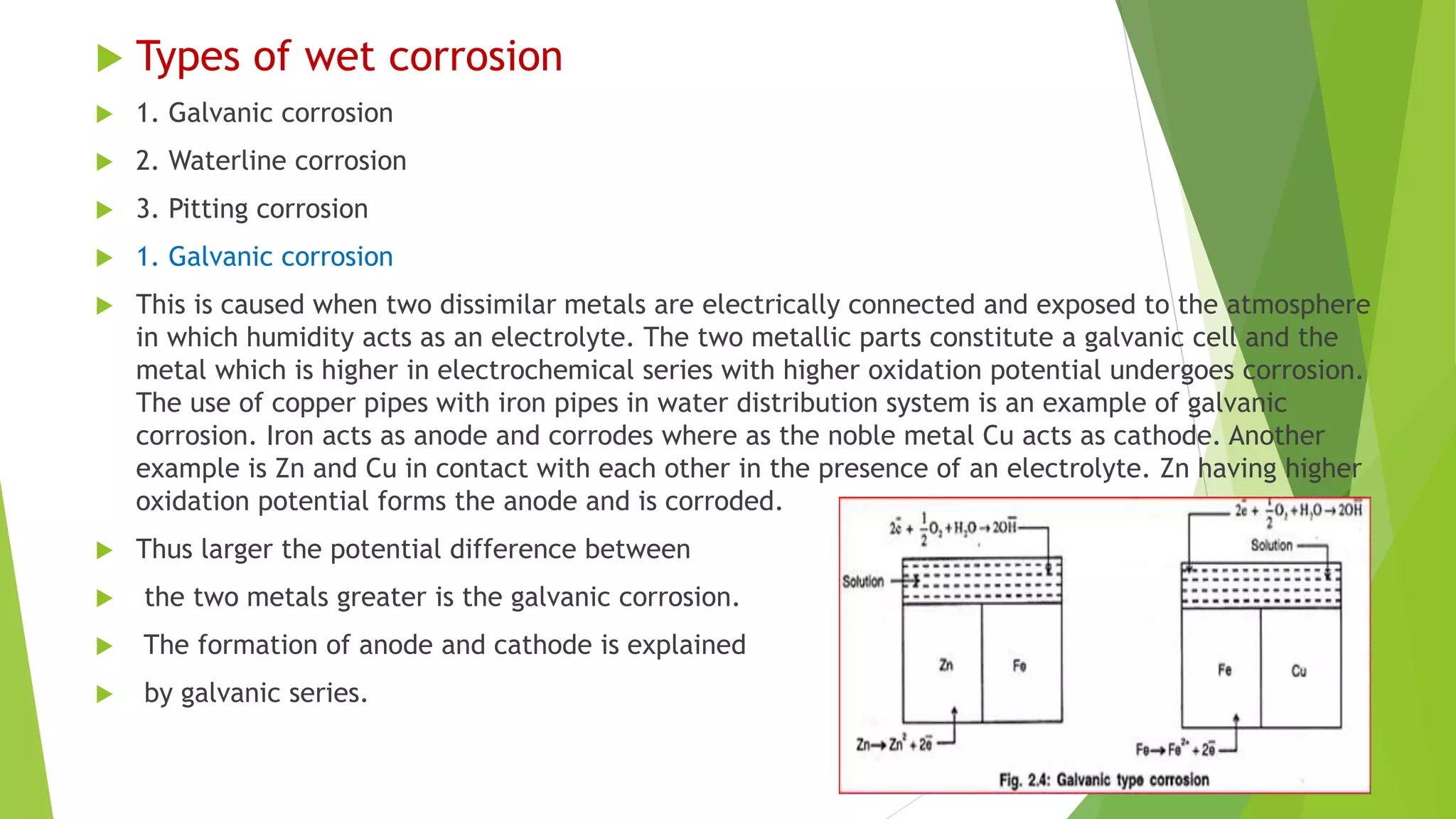
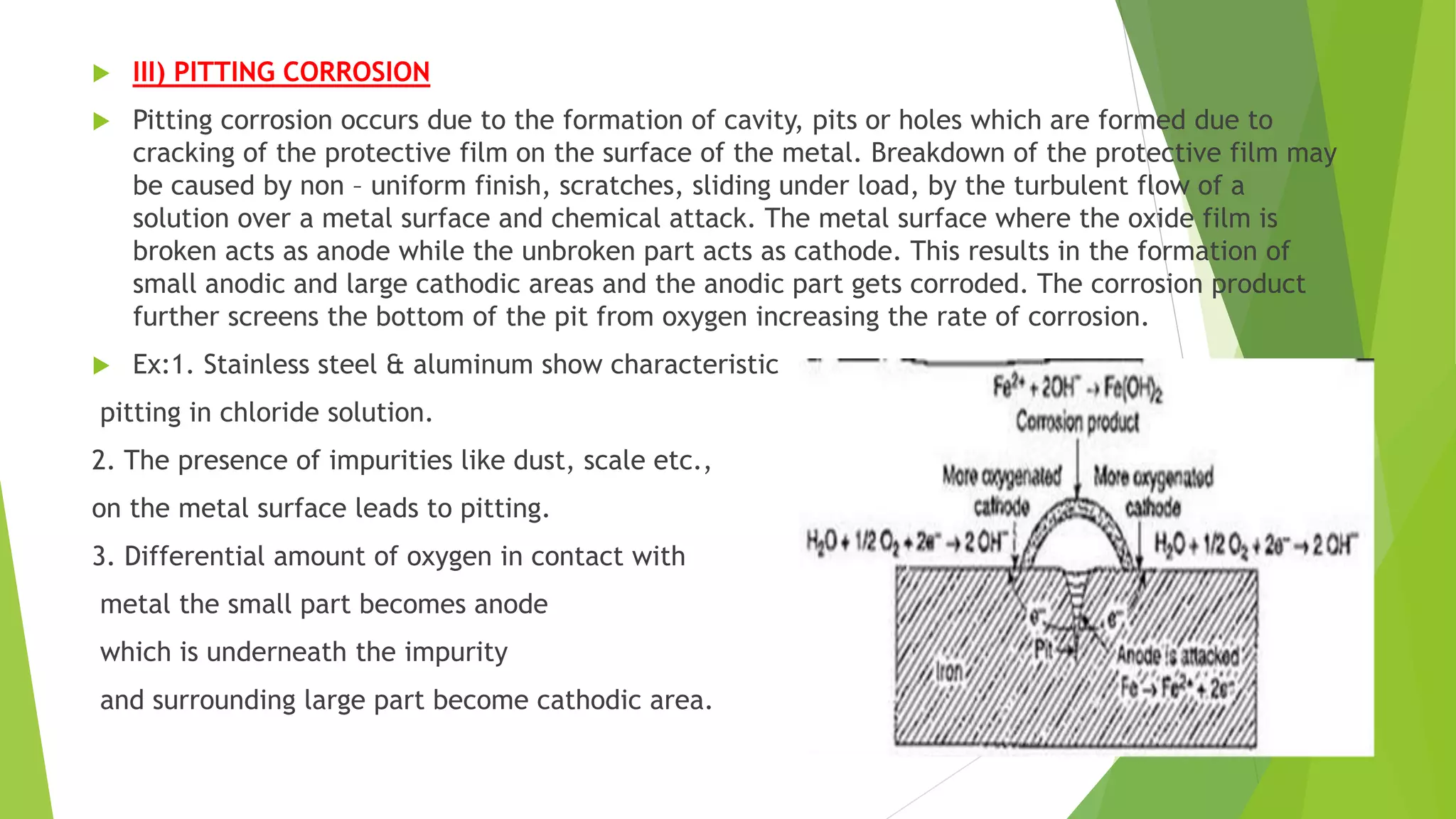
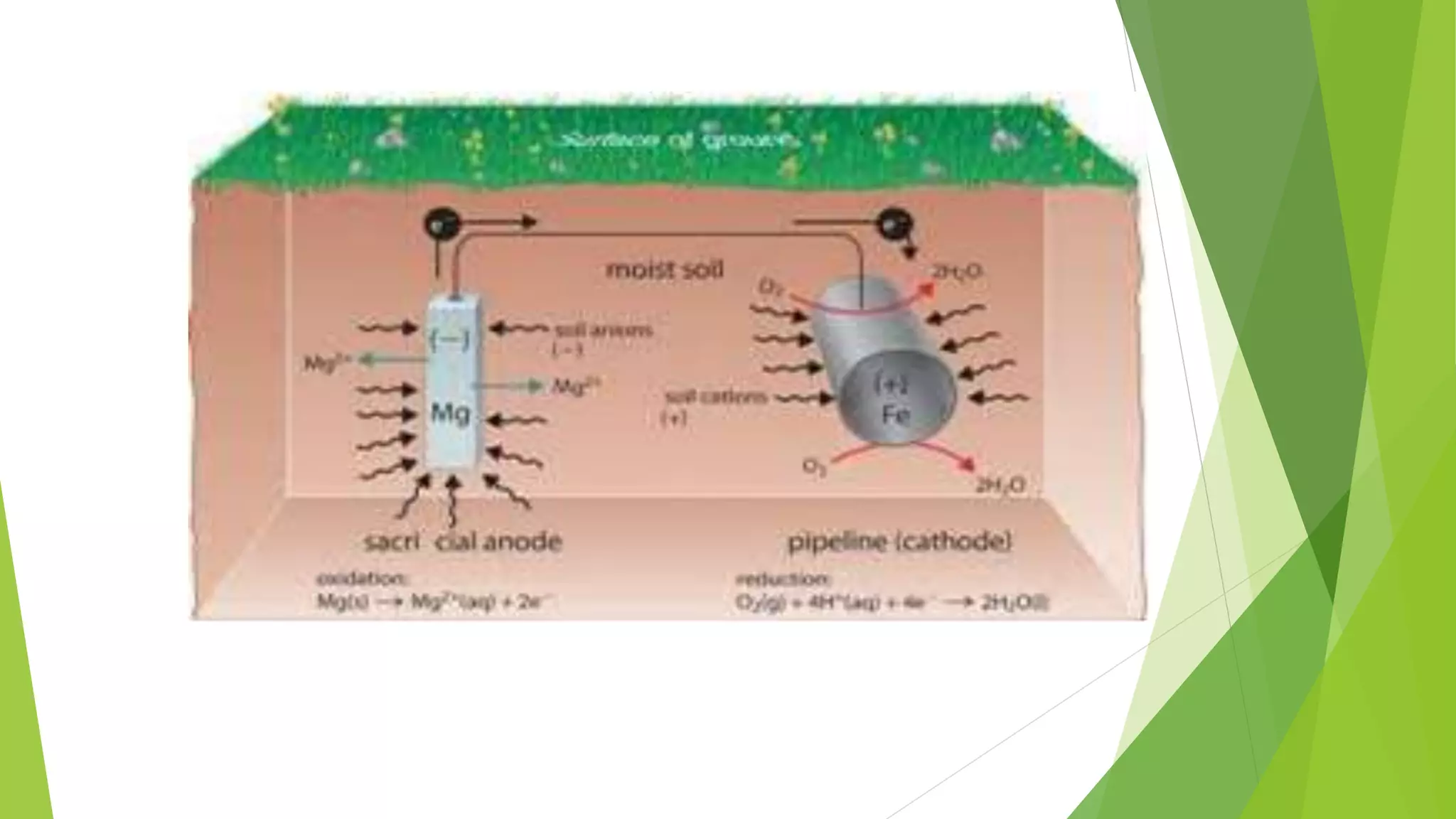
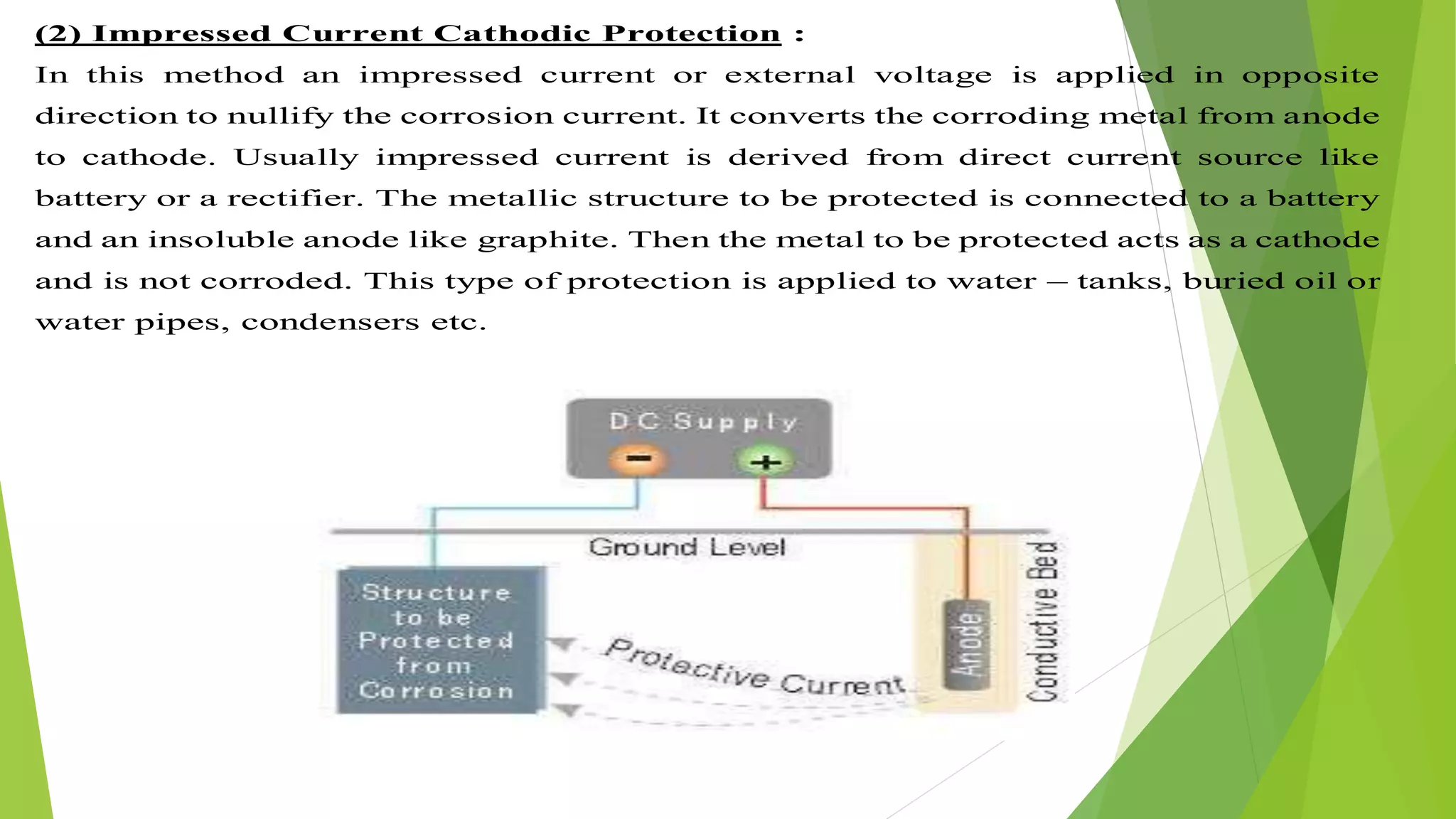
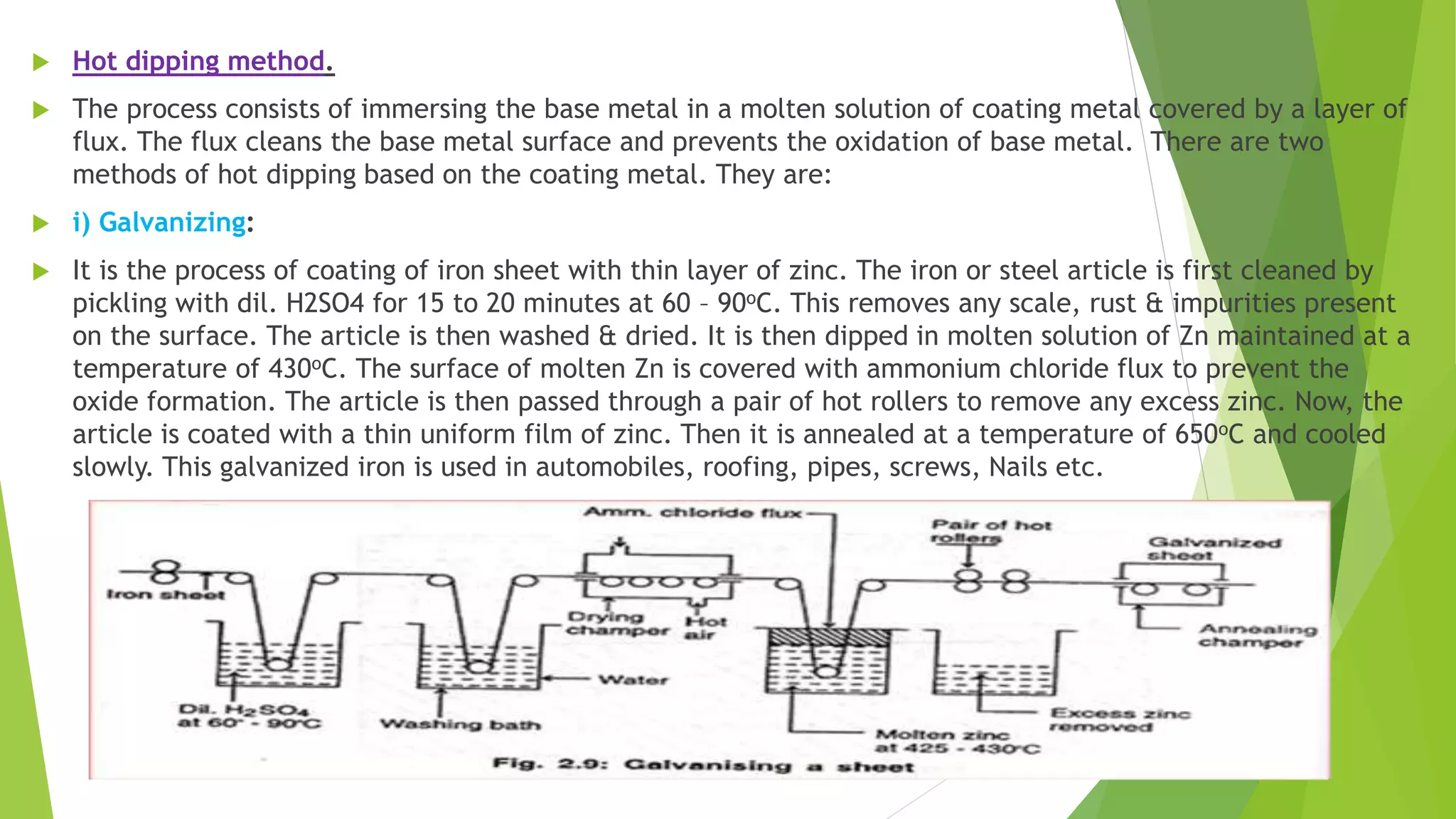
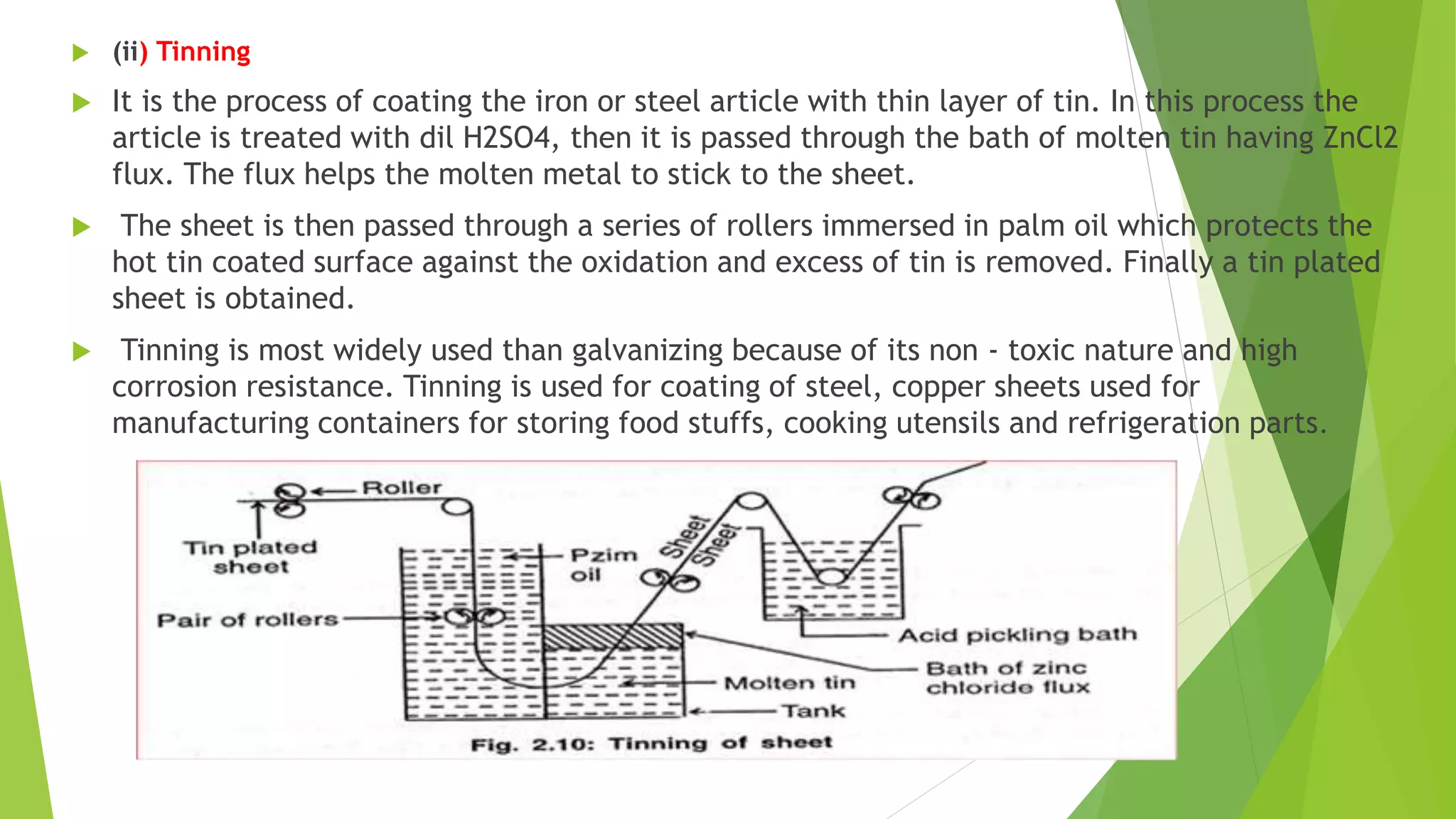
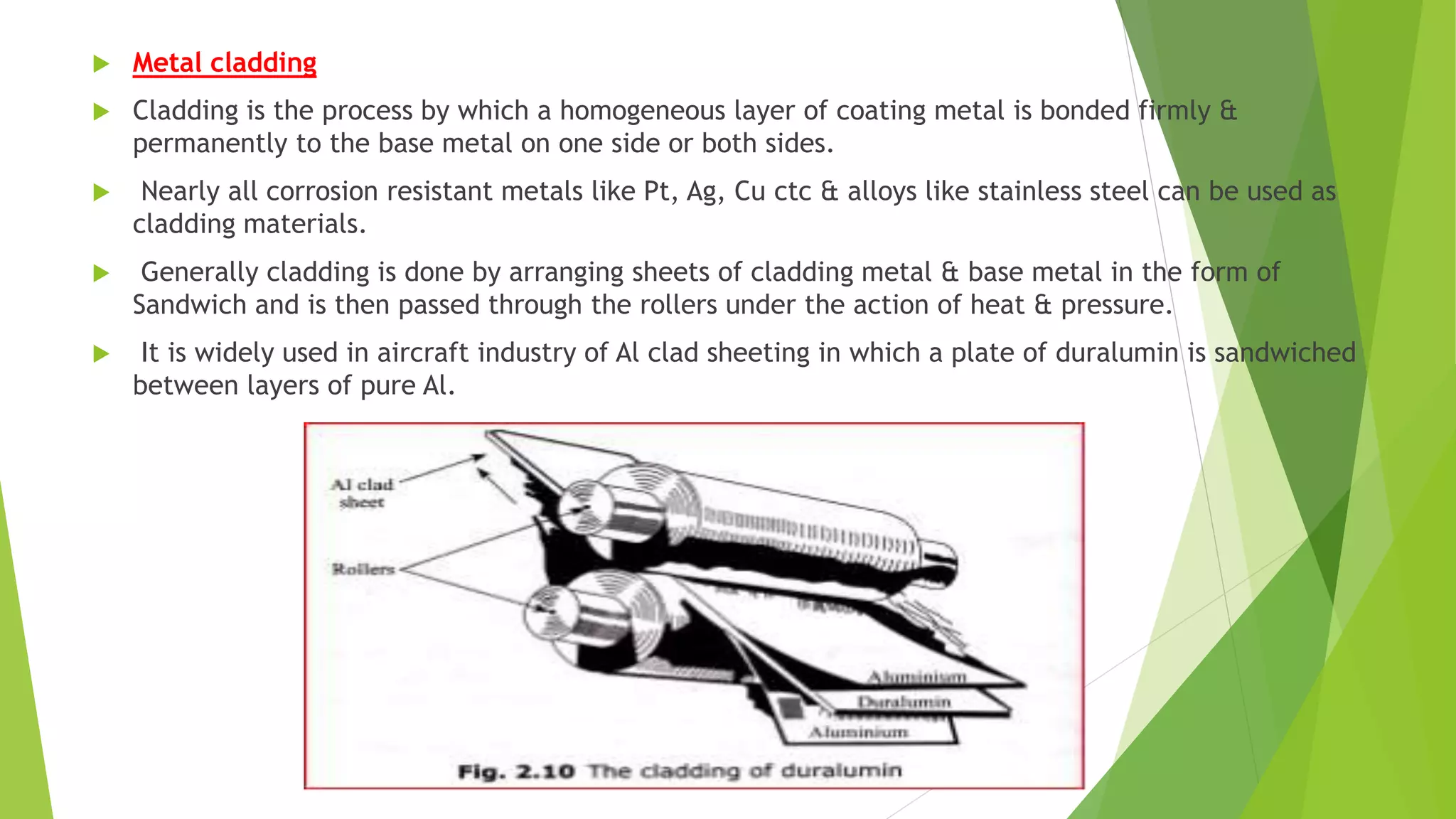
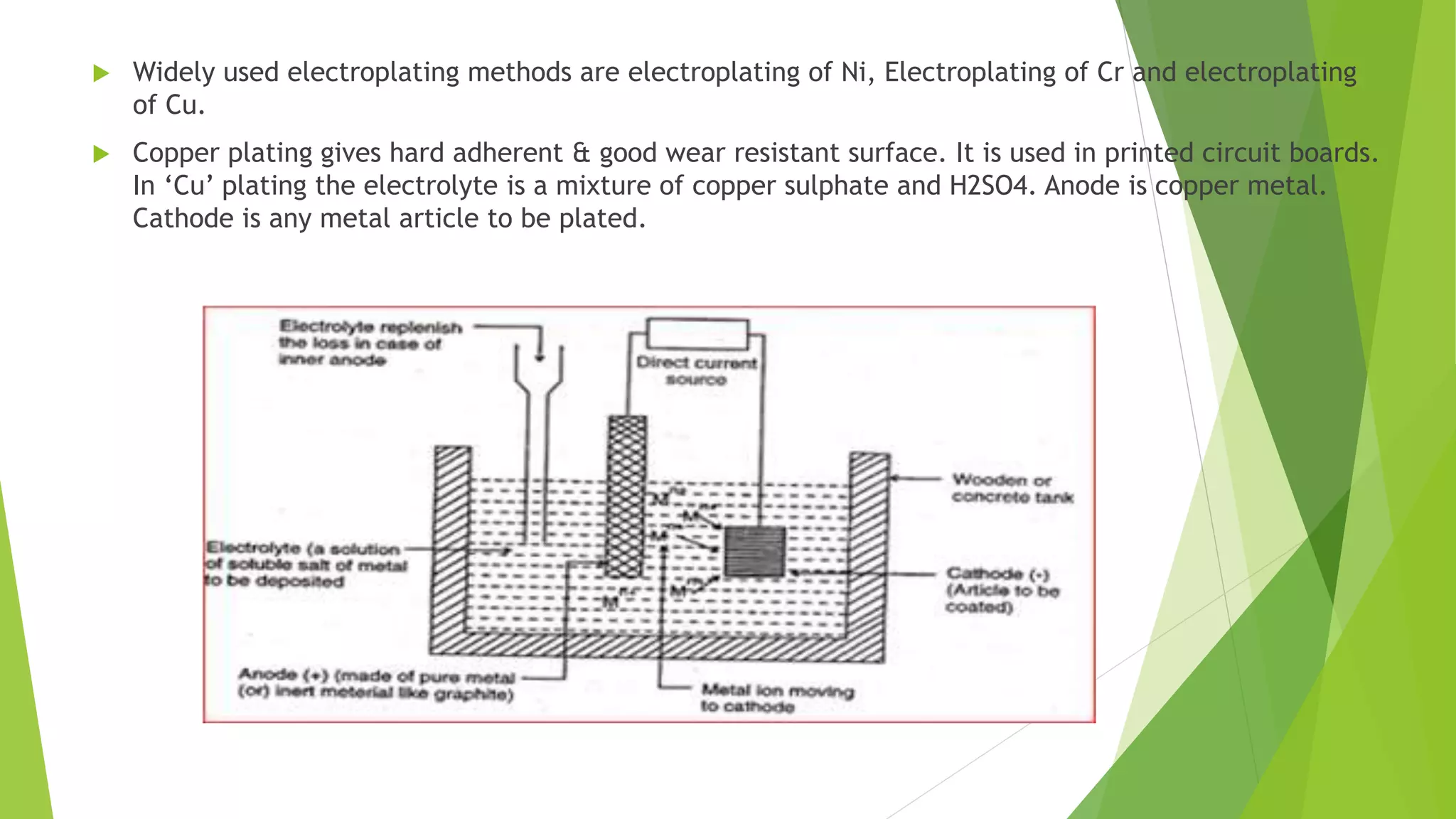
The document discusses corrosion and its causes. Corrosion occurs via chemical or electrochemical reactions between a metal and its environment that cause deterioration. It can be caused by oxygen, hydrogen, electrical currents, stress, or bacteria. Corrosion occurs via dry/chemical reactions directly with gases or wet/electrochemical reactions in an electrolyte that form anodes and cathodes. The rate depends on factors like the metal's position in the galvanic series and properties of any surface oxide or corrosion product layer.