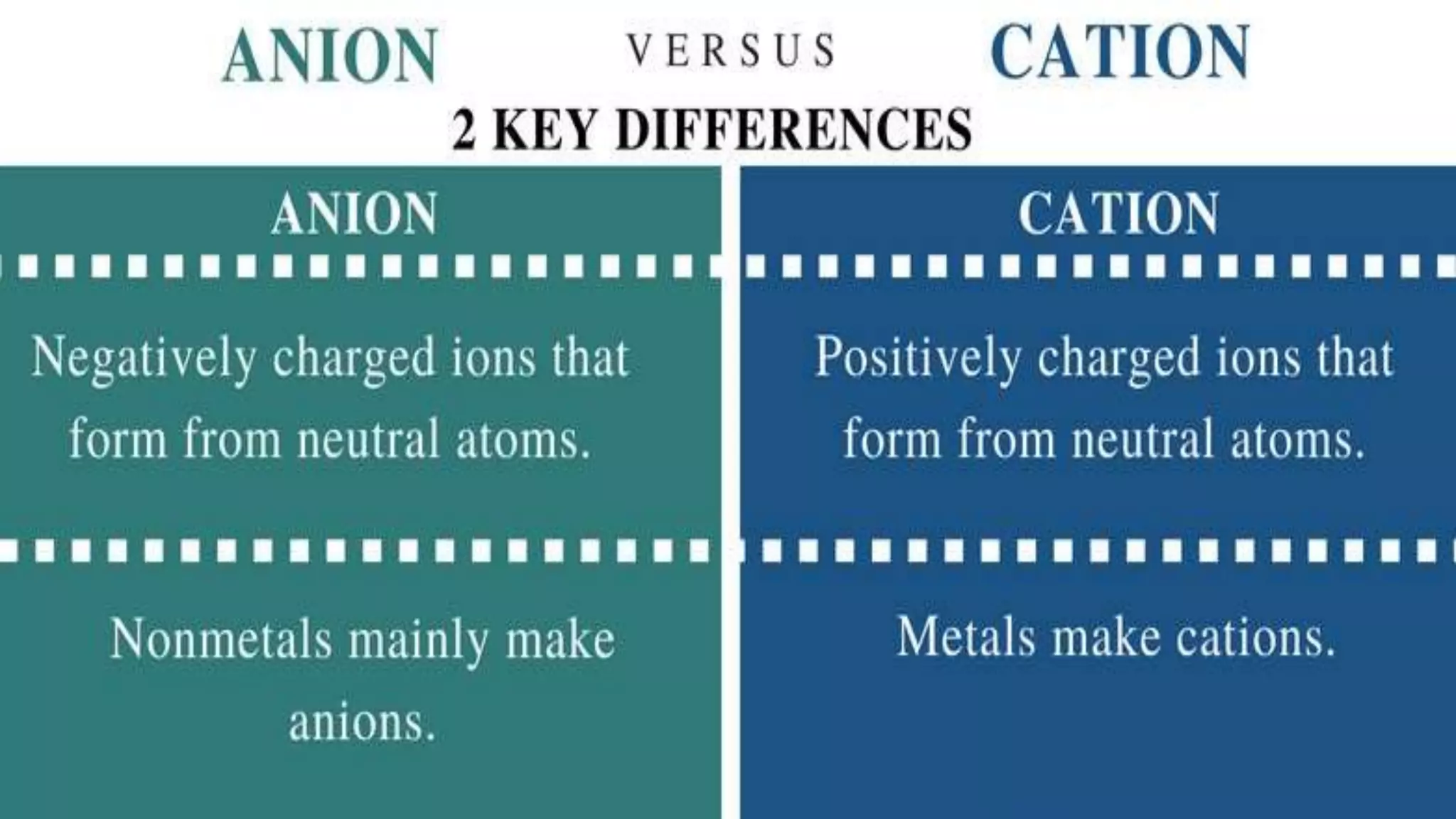
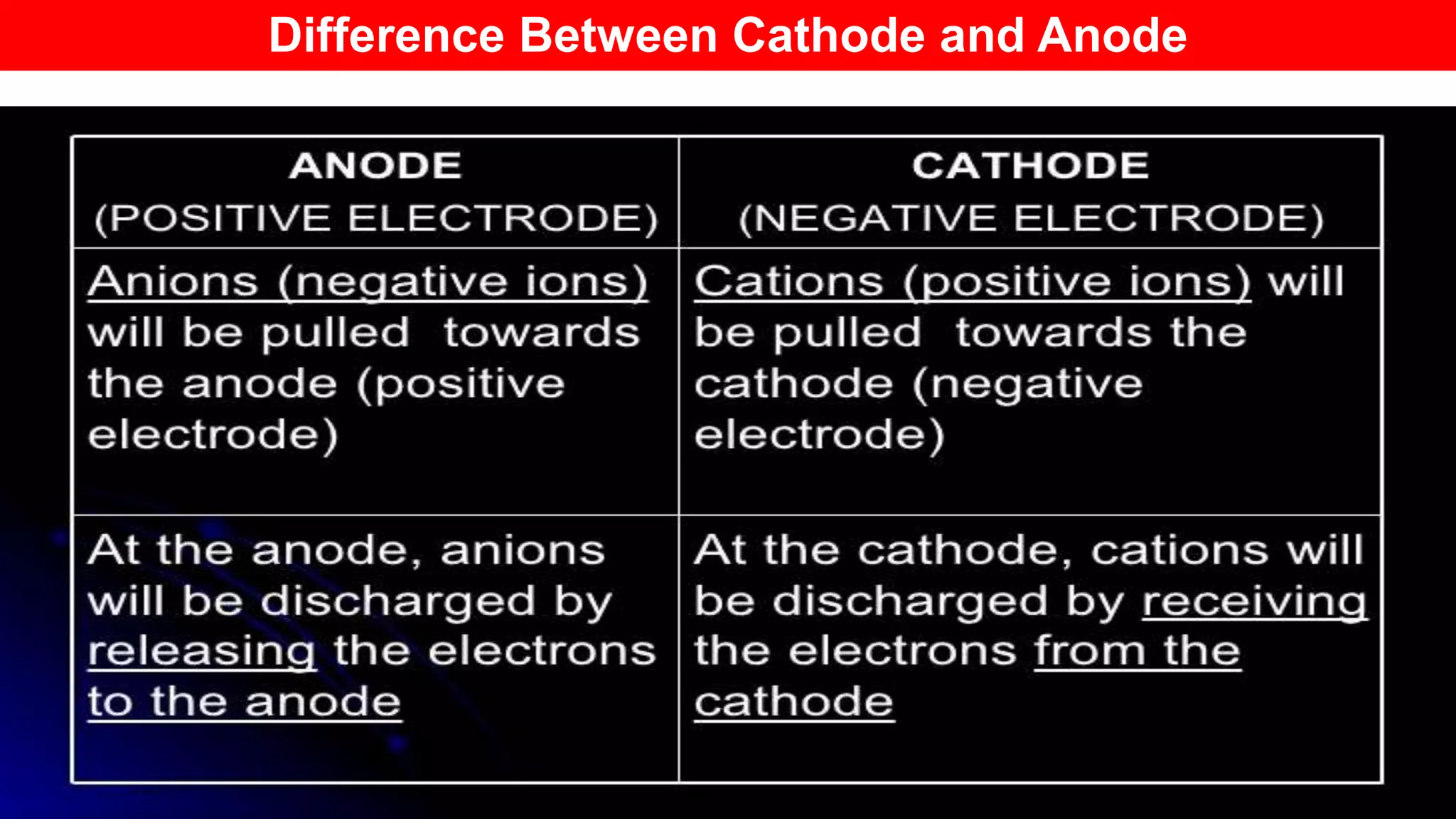
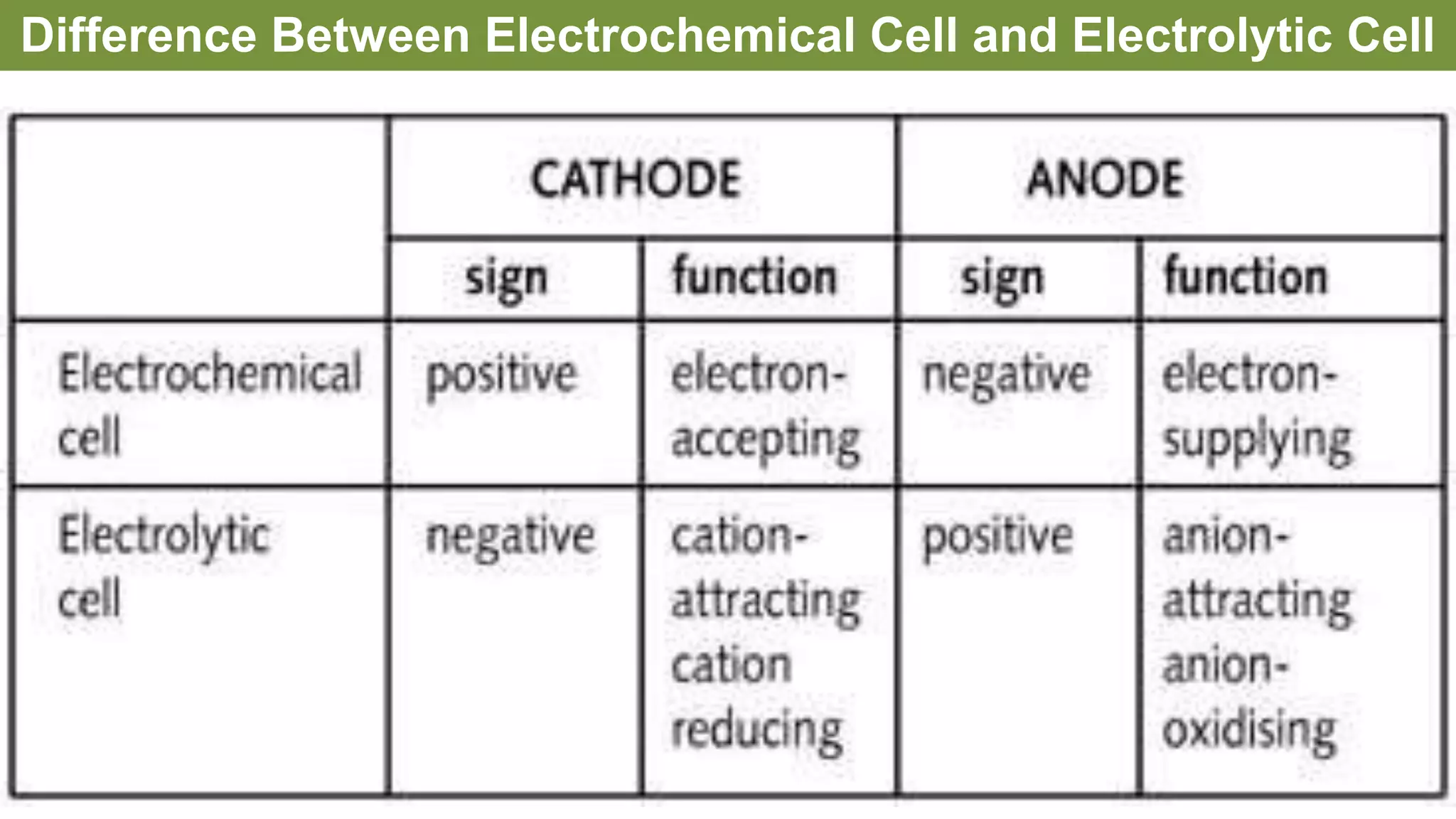
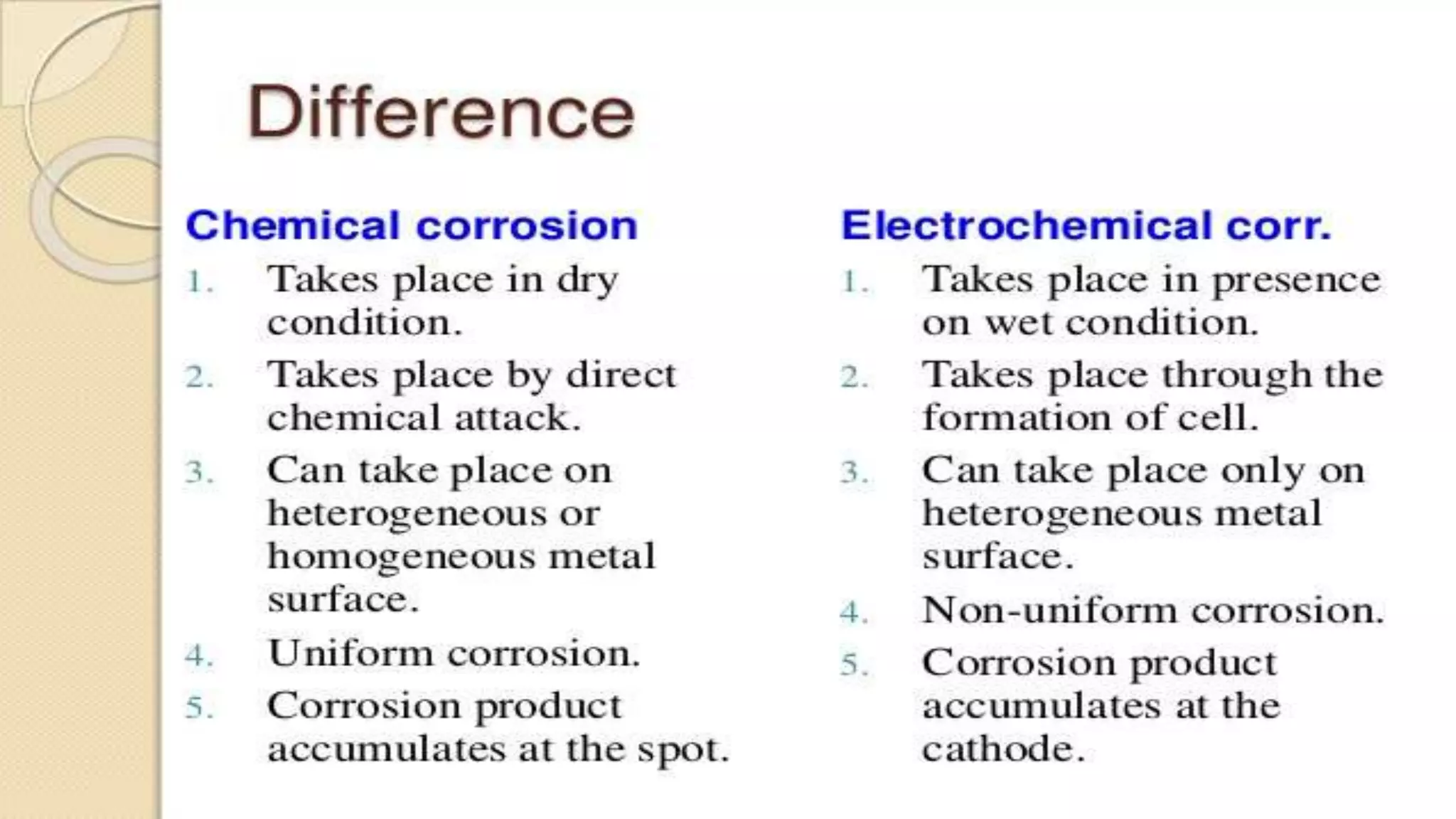
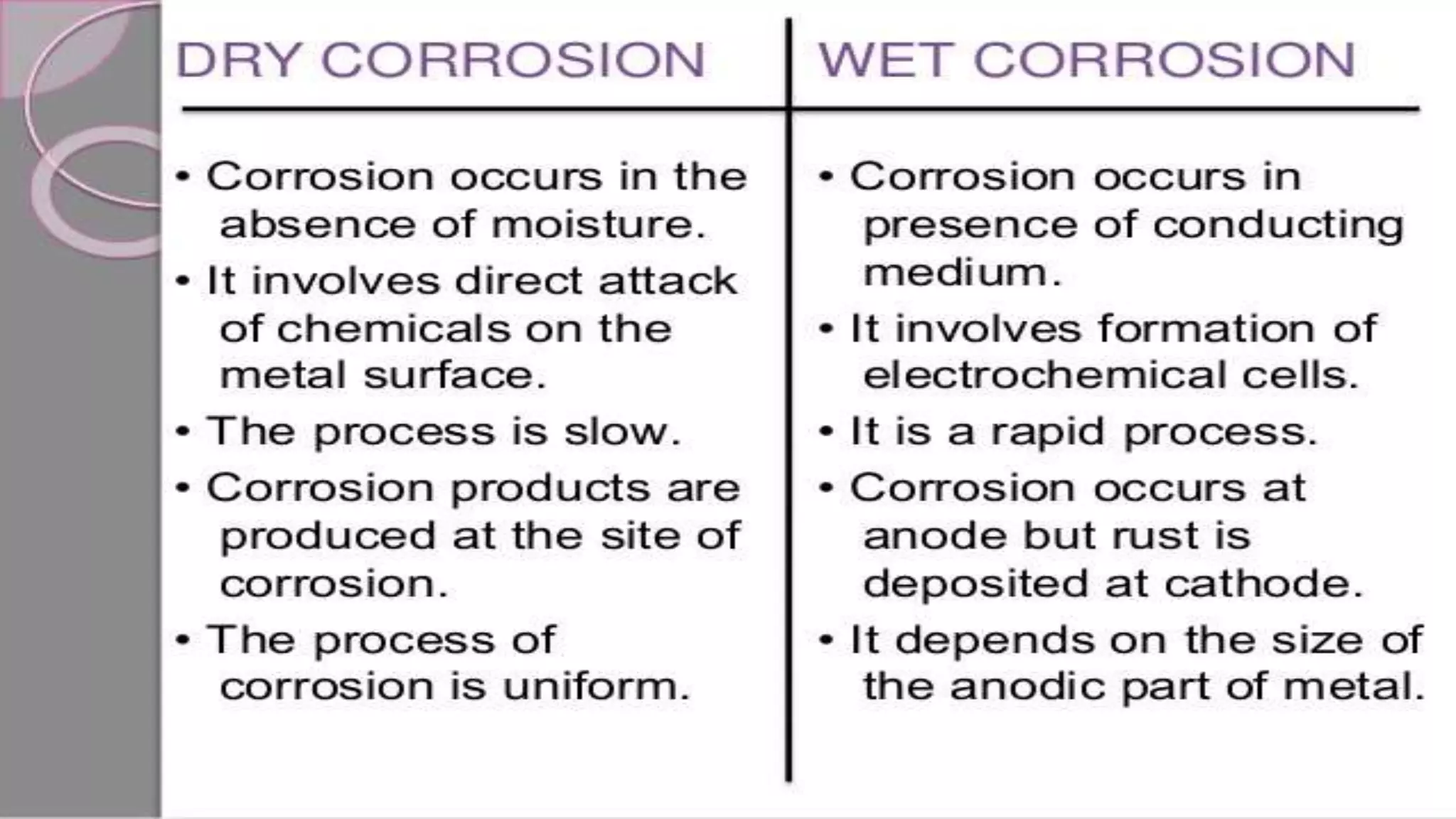
The document discusses various concepts related to corrosion and electrochemistry, including the definitions of cathode and anode, electrochemical cells versus electrolytic cells, and the electrochemical series. It elaborates on different types of corrosion such as galvanic, concentration, and pitting corrosion, as well as methods for protection against corrosion like sacrificial anodes and galvanization. Additionally, it highlights the importance of protective coatings and the process of tinning in corrosion prevention.