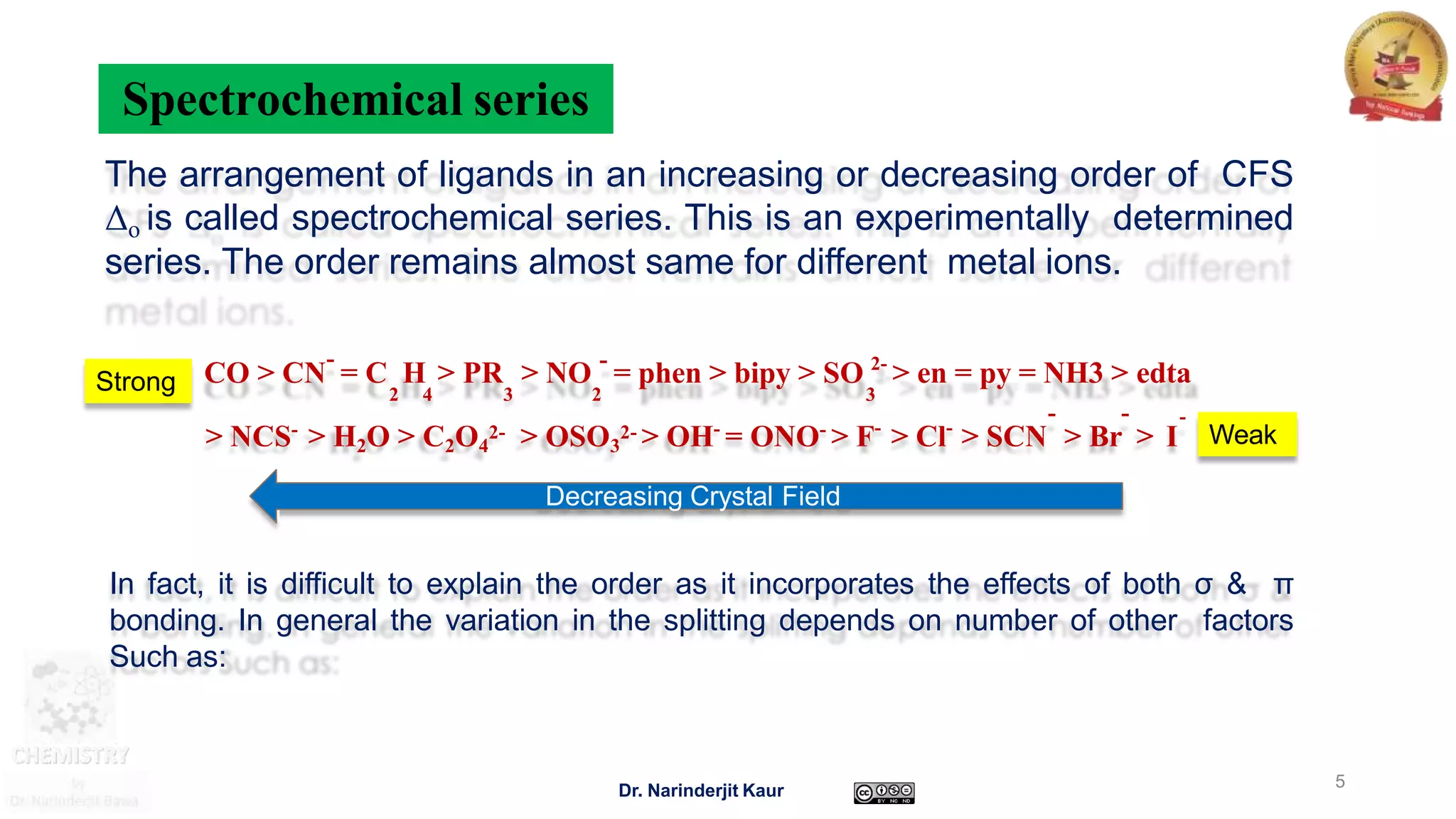
This document discusses factors that affect crystal field splitting (Δo) in transition metal complexes. The key factors are:
1) The nature of the ligands - ligands higher in the spectrochemical series (e.g. CN-) cause greater crystal field splitting than ligands lower in the series (e.g. halides).
2) The oxidation state of the transition metal ion - higher oxidation states result in greater Δo.
3) The type of d-orbitals - later transition metals in a period have larger d-orbitals resulting in greater Δo.
4) The geometry of the complex - octahedral complexes have higher Δo than tetrahedral complexes of the same metal ion and






![Which of the following would
have the largest value ofΔo?
A. [Cr(H2O)6]3+
B. [CrF6]3-
C. [Cr(NH3)6]3+
8
Dr. Narinderjit Kaur](https://image.slidesharecdn.com/narinderjitmlbfactorscfs8-201002130520/75/Factors-affecting-Crystal-Field-Splitting-by-Dr-Narinderjit-Kaur-8-2048.jpg)
![6 2 3For [Co(III)L ], Δ in cm-1: 13,100 (F-
); 20,760 (H O); 22,870 (NH )
For [Cr(III)L ], Δ in cm-1: 15,260 (F-
); 17,830 (H O); 26,280 (CN-
)
6 2
Complex ligand Donor atom Δ in cm-1
[CrCl6]3- Cl-
Cl 13640
[CrF6]3- F-
F 15260
[Cr(H2O)6]3+ H2O O 17830
[Cr(NH3)6]3+ NH3 N 21680
[Cr(en)3]3+ en N 21900
[Cr(CN-
)6]3- CN-
C 26280
9
Dr. Narinderjit Kaur](https://image.slidesharecdn.com/narinderjitmlbfactorscfs8-201002130520/75/Factors-affecting-Crystal-Field-Splitting-by-Dr-Narinderjit-Kaur-9-2048.jpg)

![2. Oxidation State of Metal ion
Δo =10,200 cm-1 for[Co(NH3)6]2+
Δo =22,870 cm-1 for[Co(NH3)6]3+
Δo =32,200 cm-1 for [Fe(CN)6]4-
Δo =35,000 cm-1for[Fe(CN)6]3-
Let’s take an example……
Metal ion with higher oxidation state causes largerΔo
As the oxidation state of the transition metal (the charge on the metal) is increased, the
surrounding ligands are attracted more closely to the metal centre. The orbitals on the
ligands interact more strongly with the d orbitals andΔo increases.
11
Dr. Narinderjit Kaur](https://image.slidesharecdn.com/narinderjitmlbfactorscfs8-201002130520/75/Factors-affecting-Crystal-Field-Splitting-by-Dr-Narinderjit-Kaur-11-2048.jpg)
![Which of the following would
have the largest value ofΔo?
A. [Fe(H2O)6]3+
B. [Fe(H2O)6]2+
12
Dr. Narinderjit Kaur](https://image.slidesharecdn.com/narinderjitmlbfactorscfs8-201002130520/75/Factors-affecting-Crystal-Field-Splitting-by-Dr-Narinderjit-Kaur-12-2048.jpg)

![3. Type of d-orbitals (transition metals)
-1
Δo = 22,870 cm (Co)
Δo = 34,100 cm-1
(Rh)
oΔ = 41,200 cm-1
(Ir)
In groups, heavier analogues have larger Δ.
For hexaammine complexes[MIII(NH3)6]3+
14
Dr. Narinderjit Kaur](https://image.slidesharecdn.com/narinderjitmlbfactorscfs8-201002130520/75/Factors-affecting-Crystal-Field-Splitting-by-Dr-Narinderjit-Kaur-14-2048.jpg)


![Complex Oxidation
state of
metal
Geometry Δ in cm-1
[Co(NH3)4]2+ II Tetrahedral 59,00
[Co(NH3)6]2+ II Octahedral 10,200
VCl4 IV Tetrahedral 7,900
[VCl6]2- IV Octahedral 15,400
Thus, all tetrahedral complexes are high spin complexes.
17
Dr. Narinderjit Kaur](https://image.slidesharecdn.com/narinderjitmlbfactorscfs8-201002130520/75/Factors-affecting-Crystal-Field-Splitting-by-Dr-Narinderjit-Kaur-17-2048.jpg)
![Which of the following would have the smallest
value ofΔo?
A. [Os(OH2)6]3+
B. [Fe(OH2)6]3+
C. [Ru(OH2)6]3+
18
Dr. Narinderjit Kaur](https://image.slidesharecdn.com/narinderjitmlbfactorscfs8-201002130520/75/Factors-affecting-Crystal-Field-Splitting-by-Dr-Narinderjit-Kaur-18-2048.jpg)
