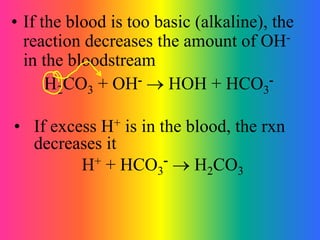Embed presentation
Download to read offline
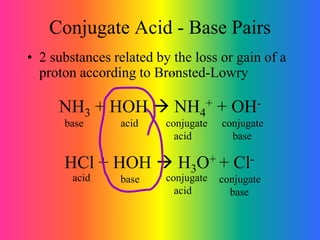

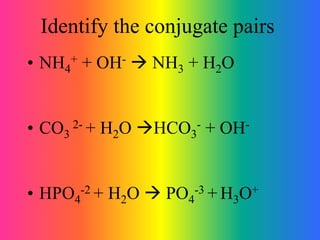



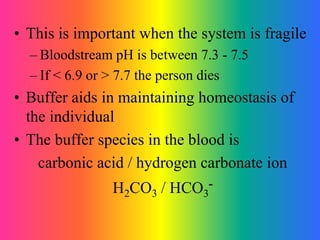
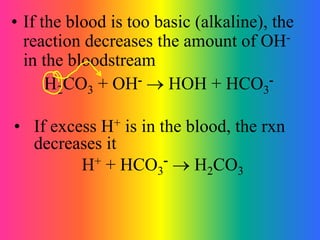


This document discusses conjugate acid-base pairs and buffer systems. It defines conjugate acid-base pairs as two substances related by the loss or gain of a proton according to Brønsted-Lowry theory. Buffers are described as mixtures that resist changes in pH when acids or bases are added. Common buffer systems include weak acids and their salts, and weak bases and their salts. The document provides examples of conjugate acid-base pairs and buffer reactions.