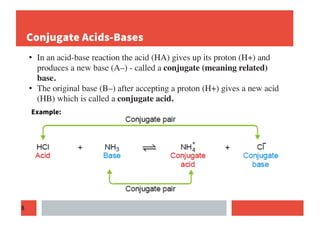
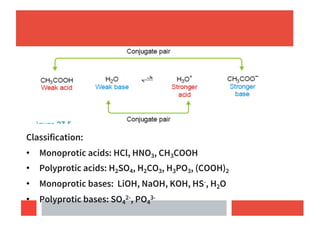
This document provides an overview of acids and bases according to different theories:
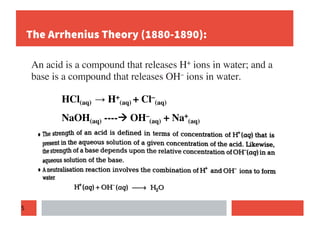
1) Arrhenius concept defines acids and bases as compounds that release H+ and OH- ions in water.
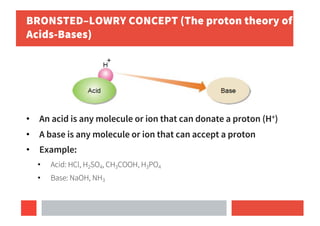
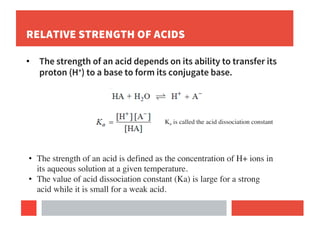
2) Bronsted-Lowry concept defines acids as proton donors and bases as proton acceptors in any reaction.
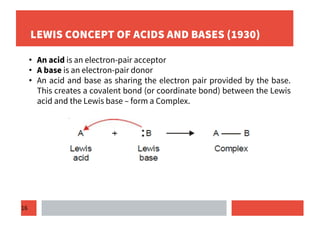
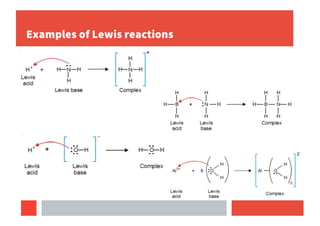
3) Lewis concept defines acids as electron pair acceptors and bases as electron pair donors, forming coordinate covalent bonds.
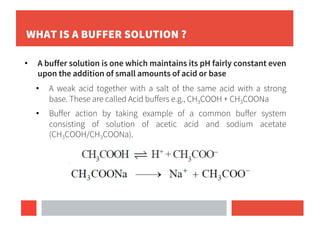
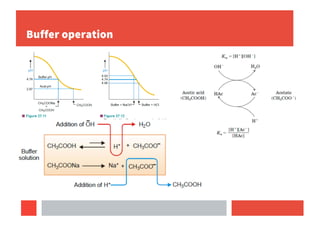
Buffer solutions maintain pH upon addition of small amounts of acid or base and are important in biological systems like blood plasma.











![21
What is pH scale?
1. Calculate the pH of 0.06 mol/L HCl.
pH = − log[0.06] = 1.22
2. Calculate the pH of 0.02 mol/L H2SO4.
pH = − log0.04 = 1.3979 = ~ 1.4
3. Calculate the pH of NaOH 0.5 mol/L.
pH = 14−(−log0.5) = ~13.7
4. If this solution is diluted 10-fold, what will be the
resulting pH?
pH = ~12.7](https://image.slidesharecdn.com/acidsandbases13052020-200512163230/85/Acids-and-bases-13052020-21-320.jpg)