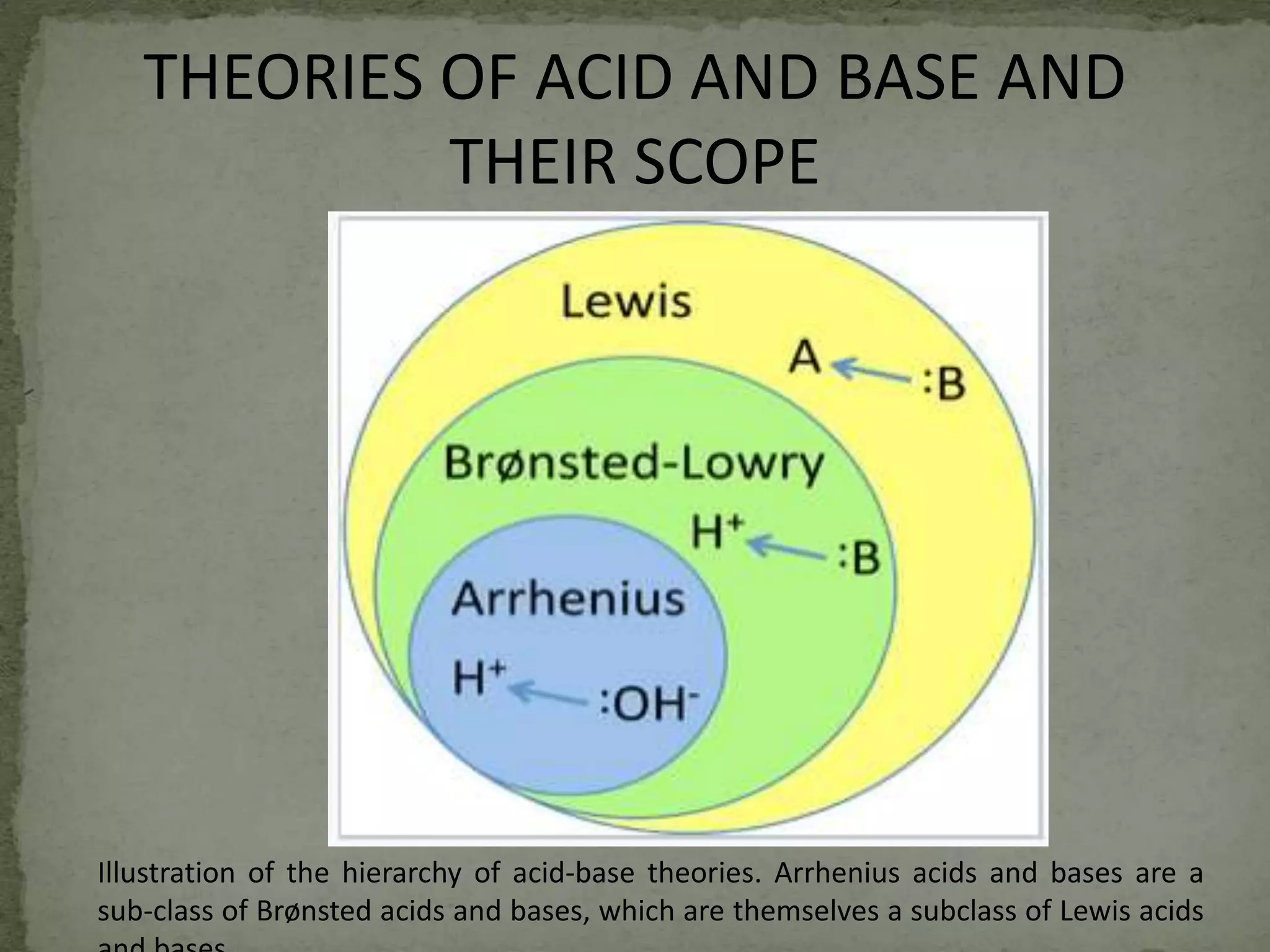
The document discusses the definitions and theories of acids and bases, focusing on the Lewis theory, which describes acids as species that accept electron pairs and bases as those that donate electron pairs. It elaborates on various examples and applications of Lewis acids and bases, including their use in chemical reactions like Friedel-Crafts alkylation. Additionally, the document mentions extended theories such as Usanovich and Lux-Flood, highlighting the broader understanding of acid-base reactions involving electron and oxide ion interactions.