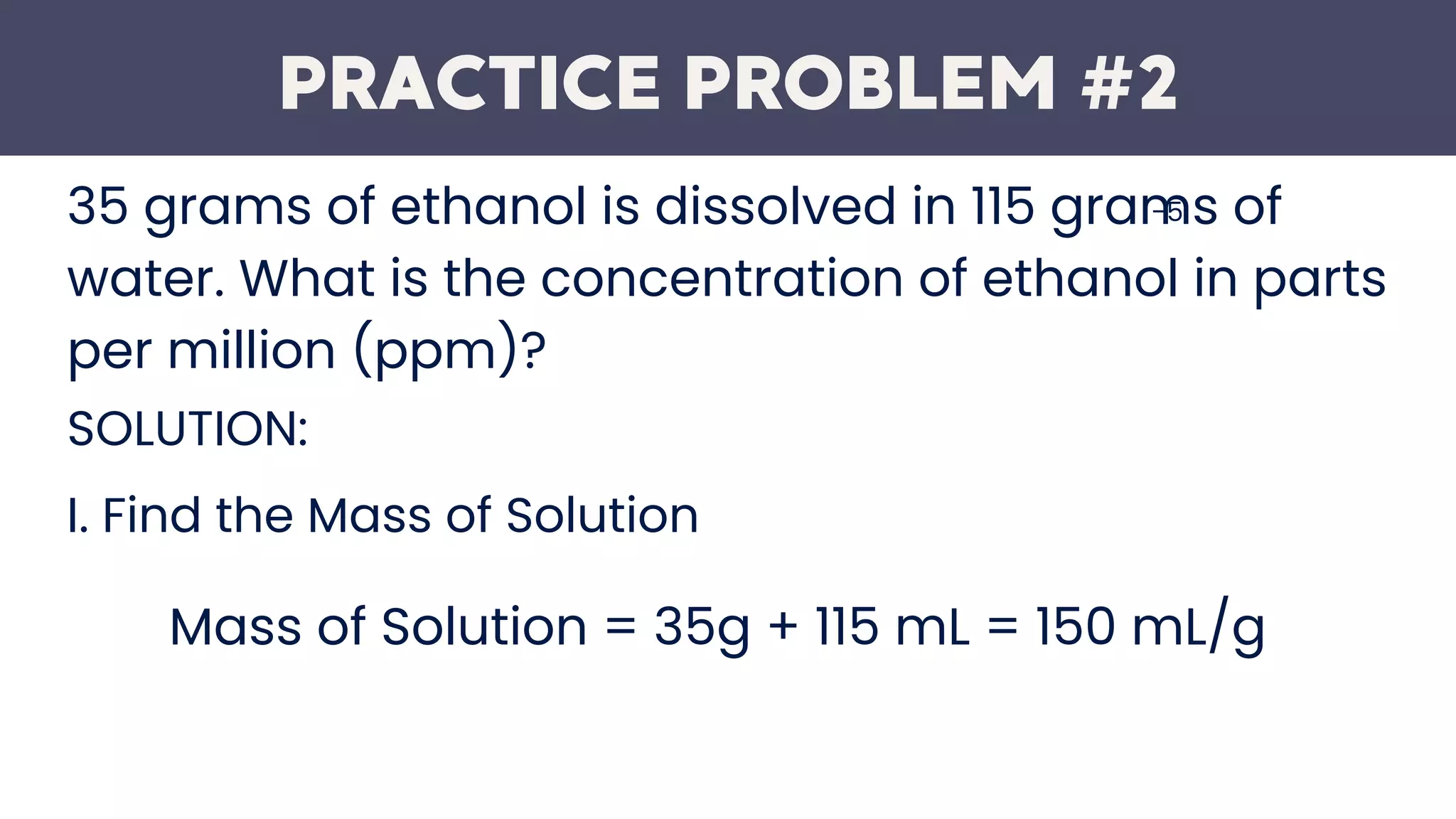
This document defines parts per million (ppm) as the number of units of mass of a contaminant per million units of total mass. It provides an example that 1 ppm in soils and sediments equals 1 mg of substance per kg of solid. The document also presents the formula for calculating ppm and works through two practice problems calculating ppm concentrations given the mass of solute and solution. It finds that the concentration of calcium ions is 76 ppm in the first example and the concentration of ethanol is 230000 ppm in the second example.