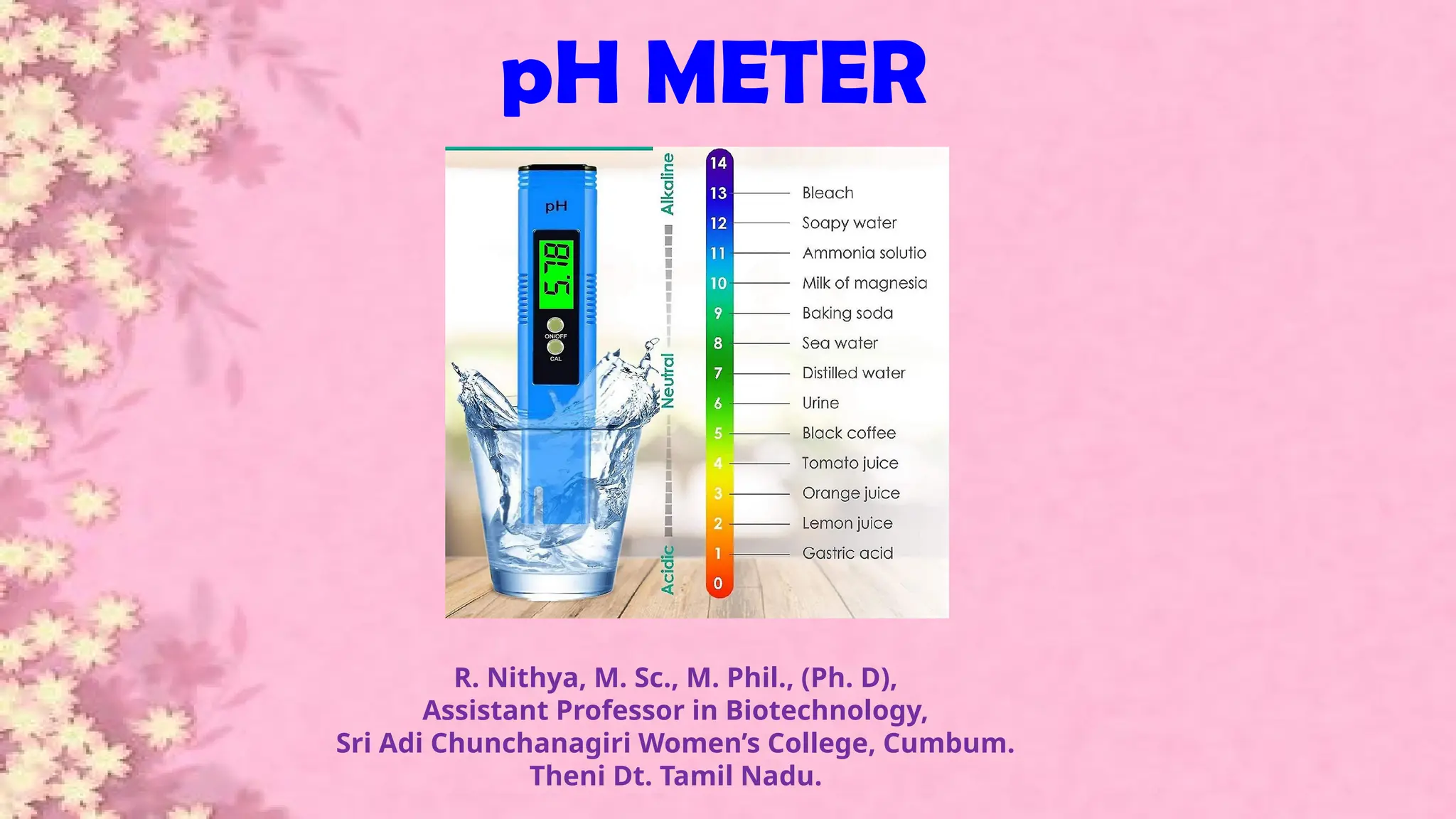
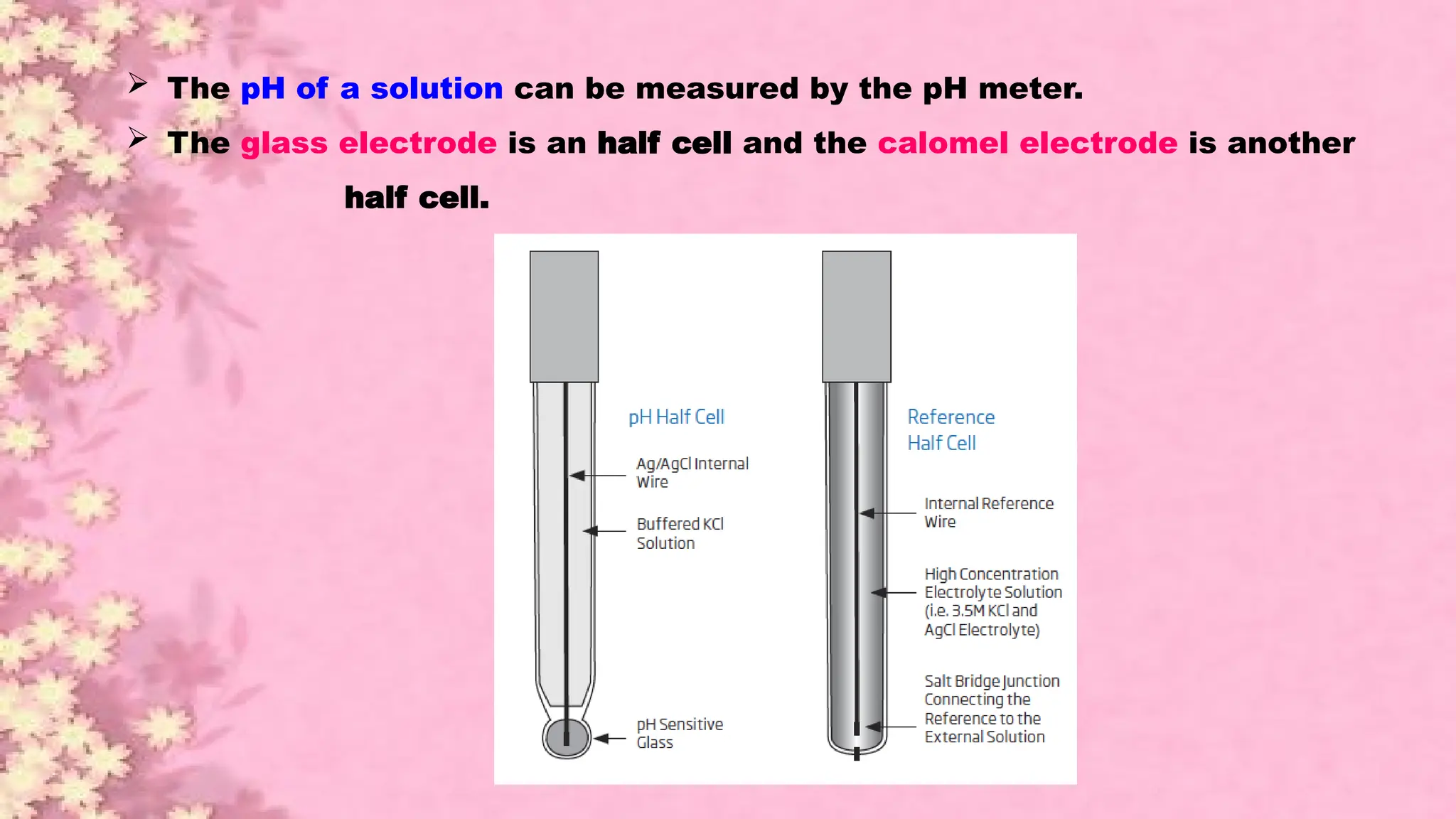
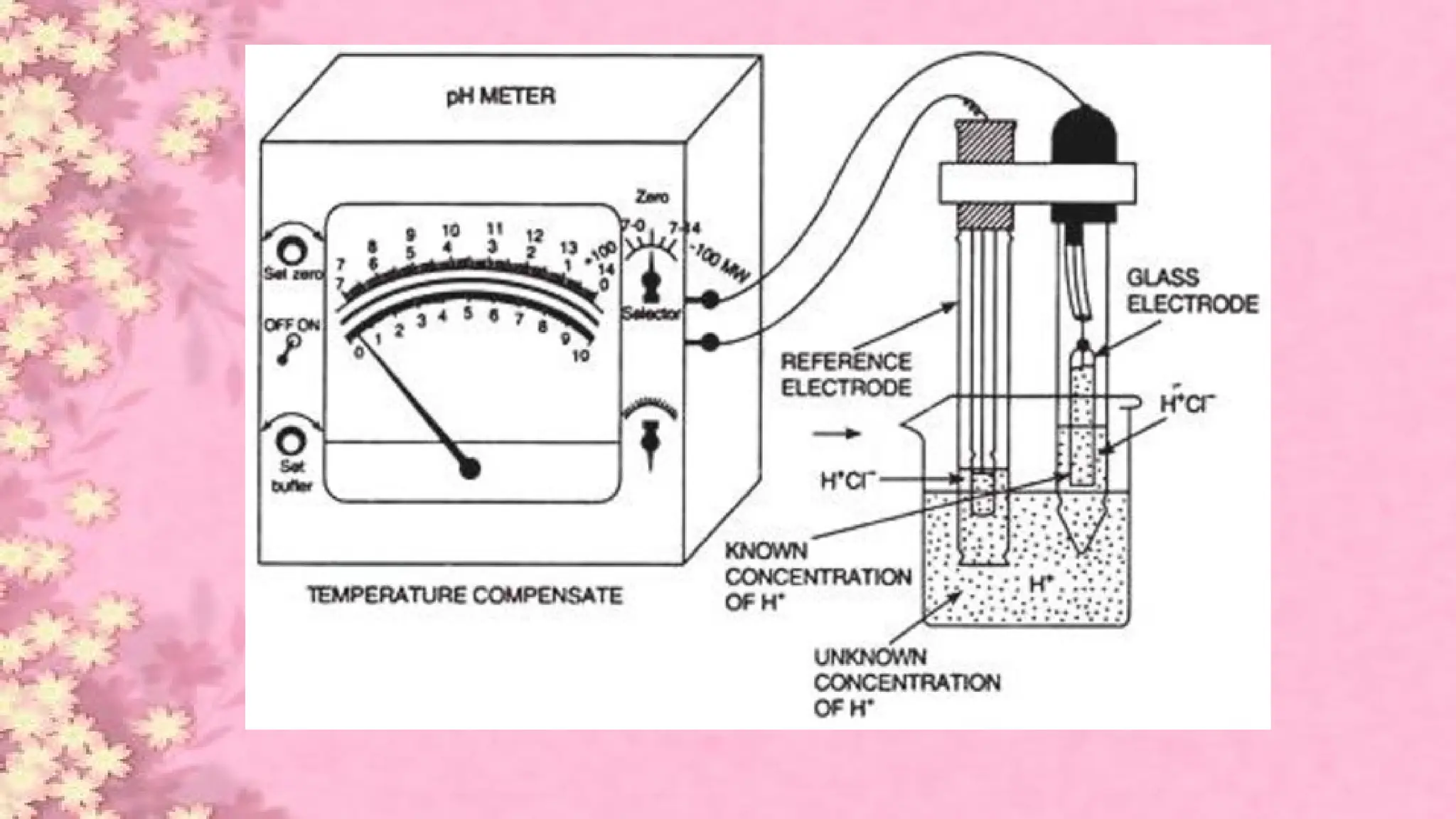
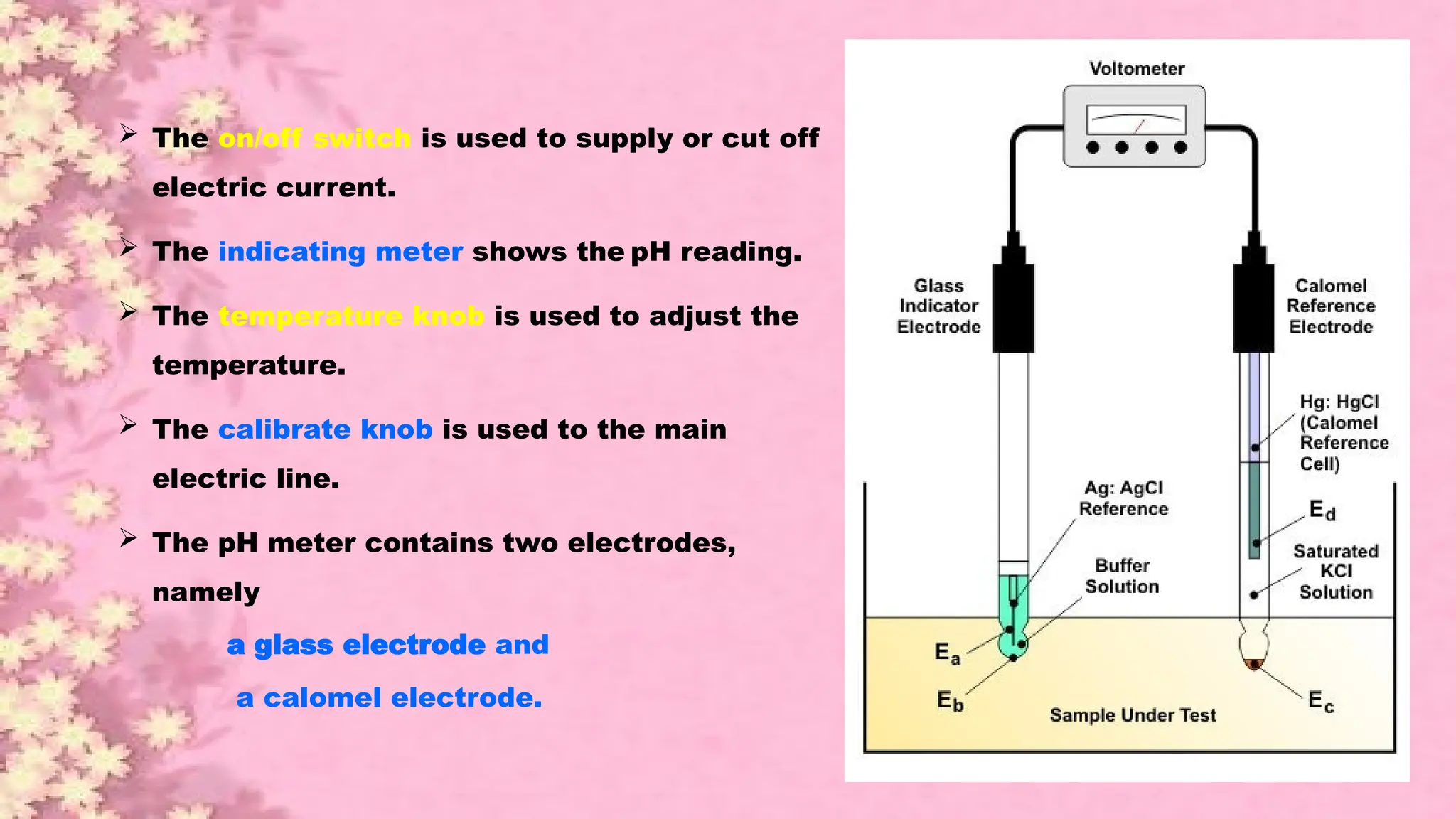
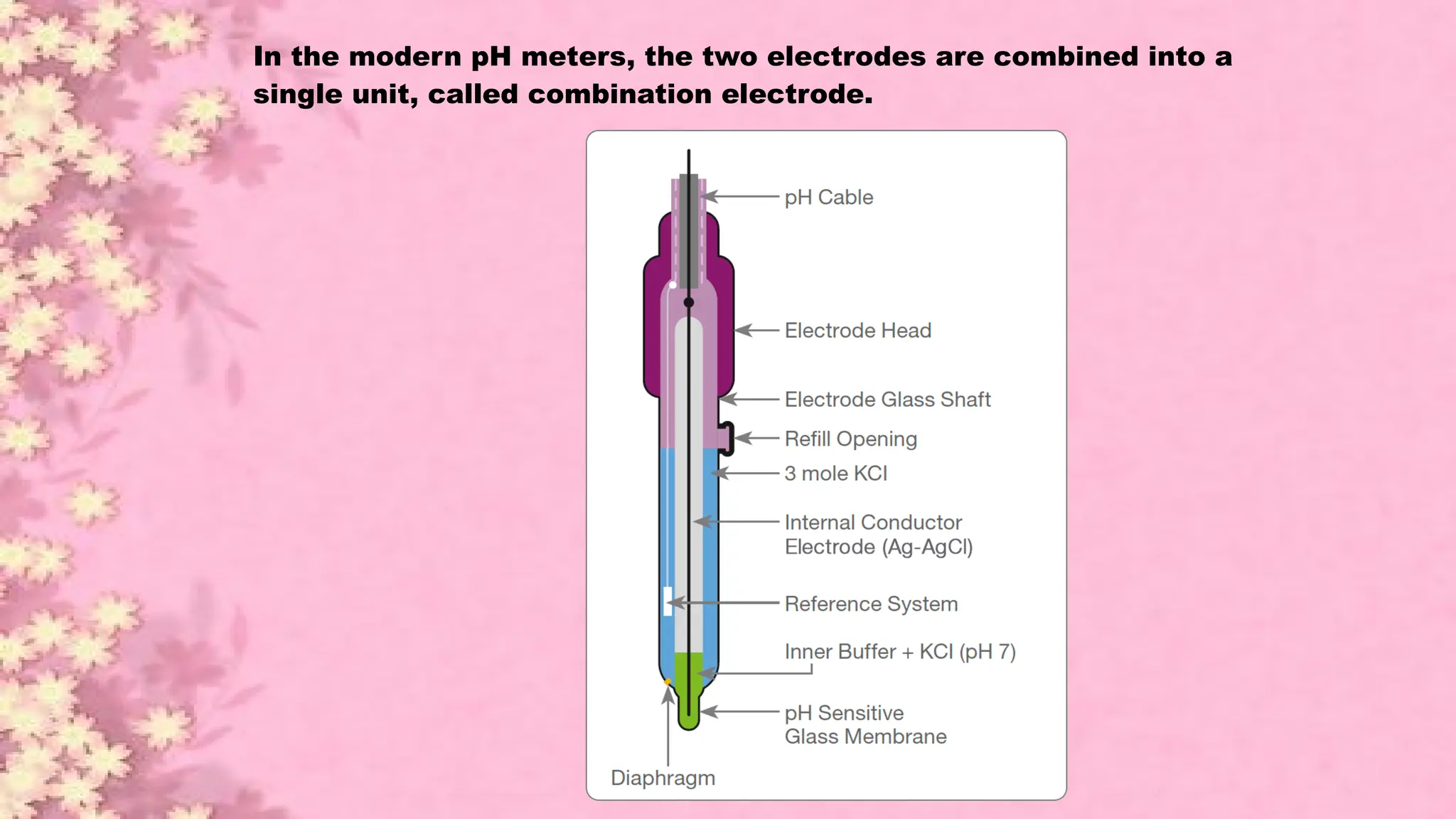
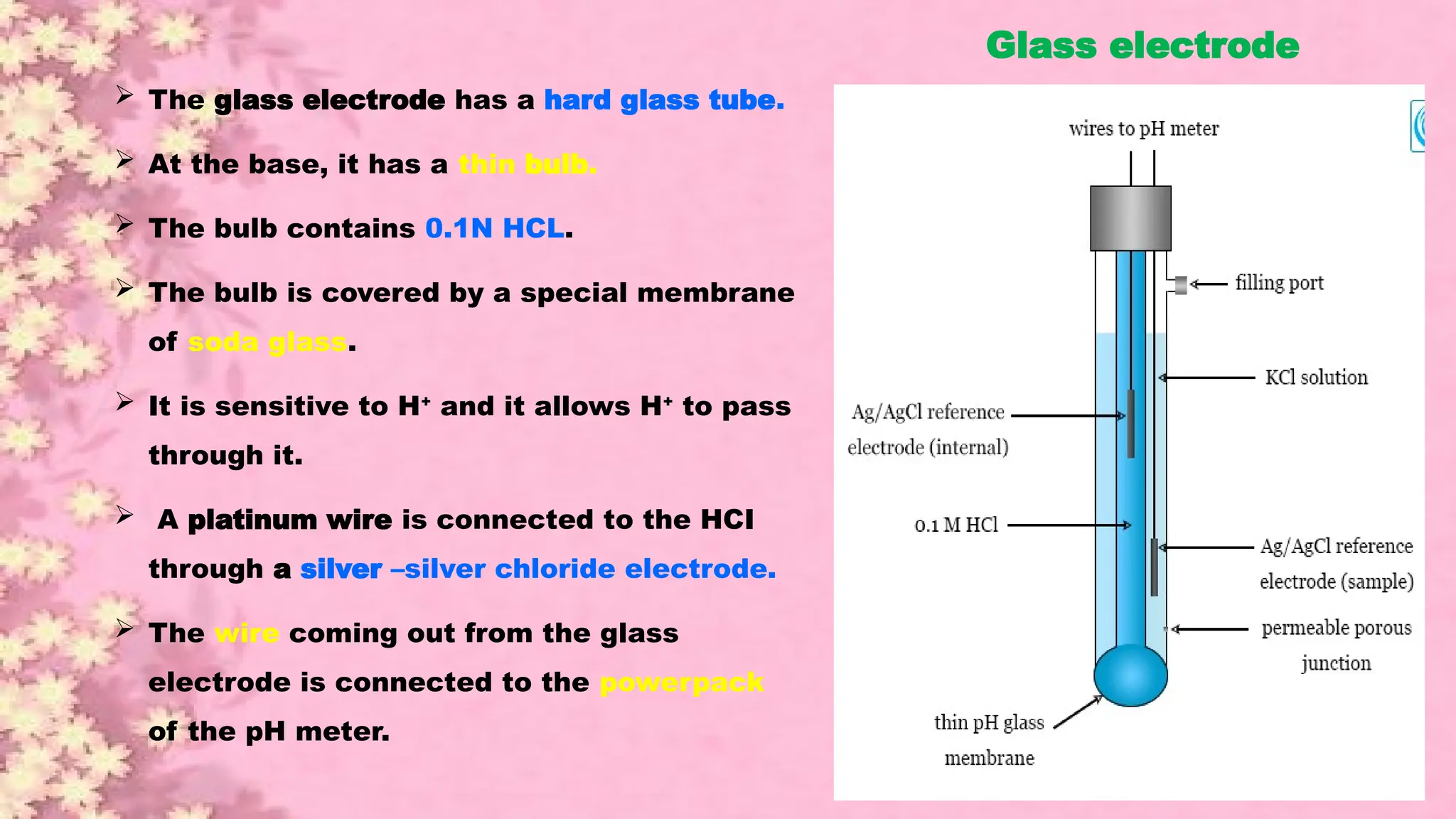
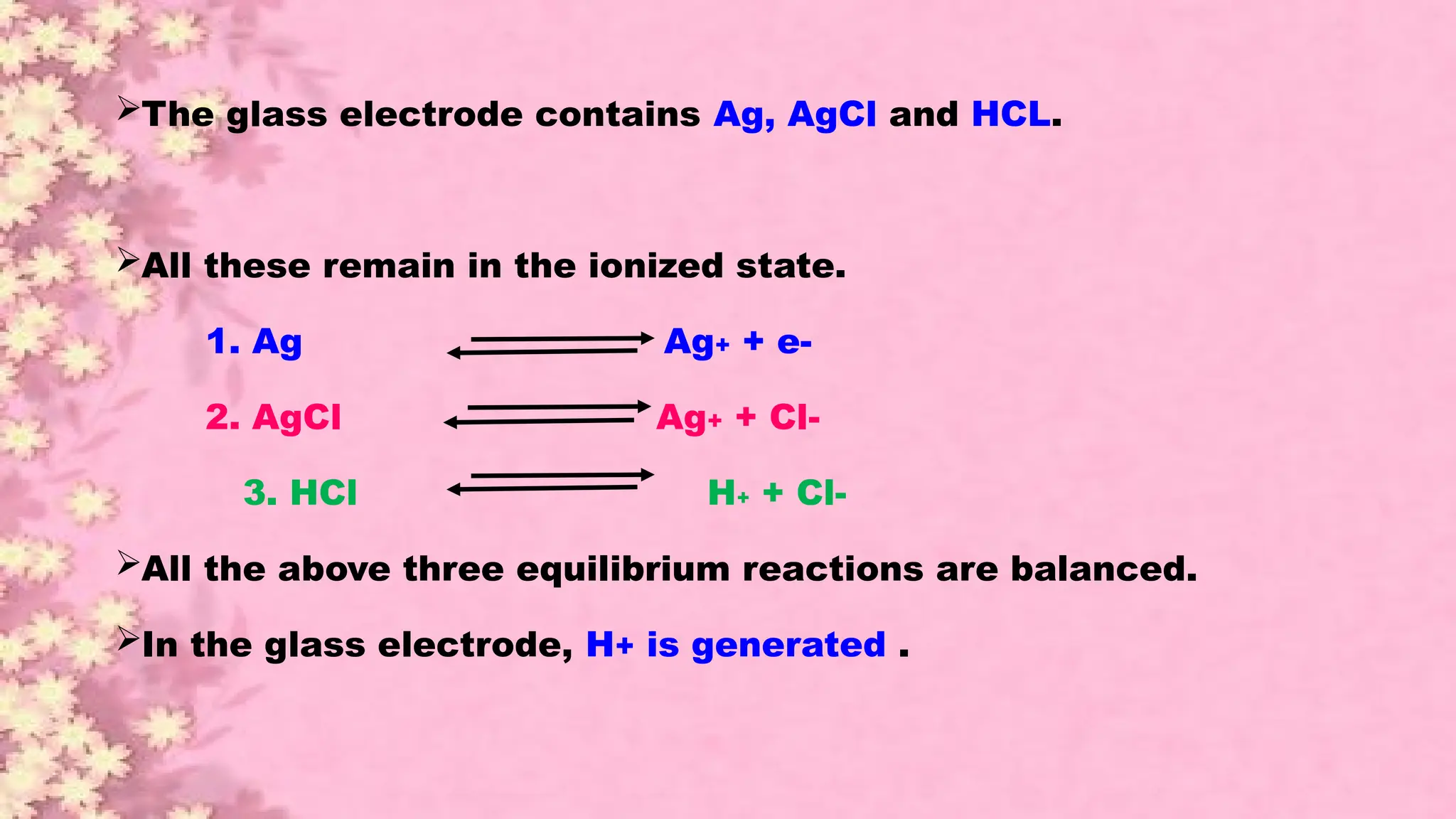
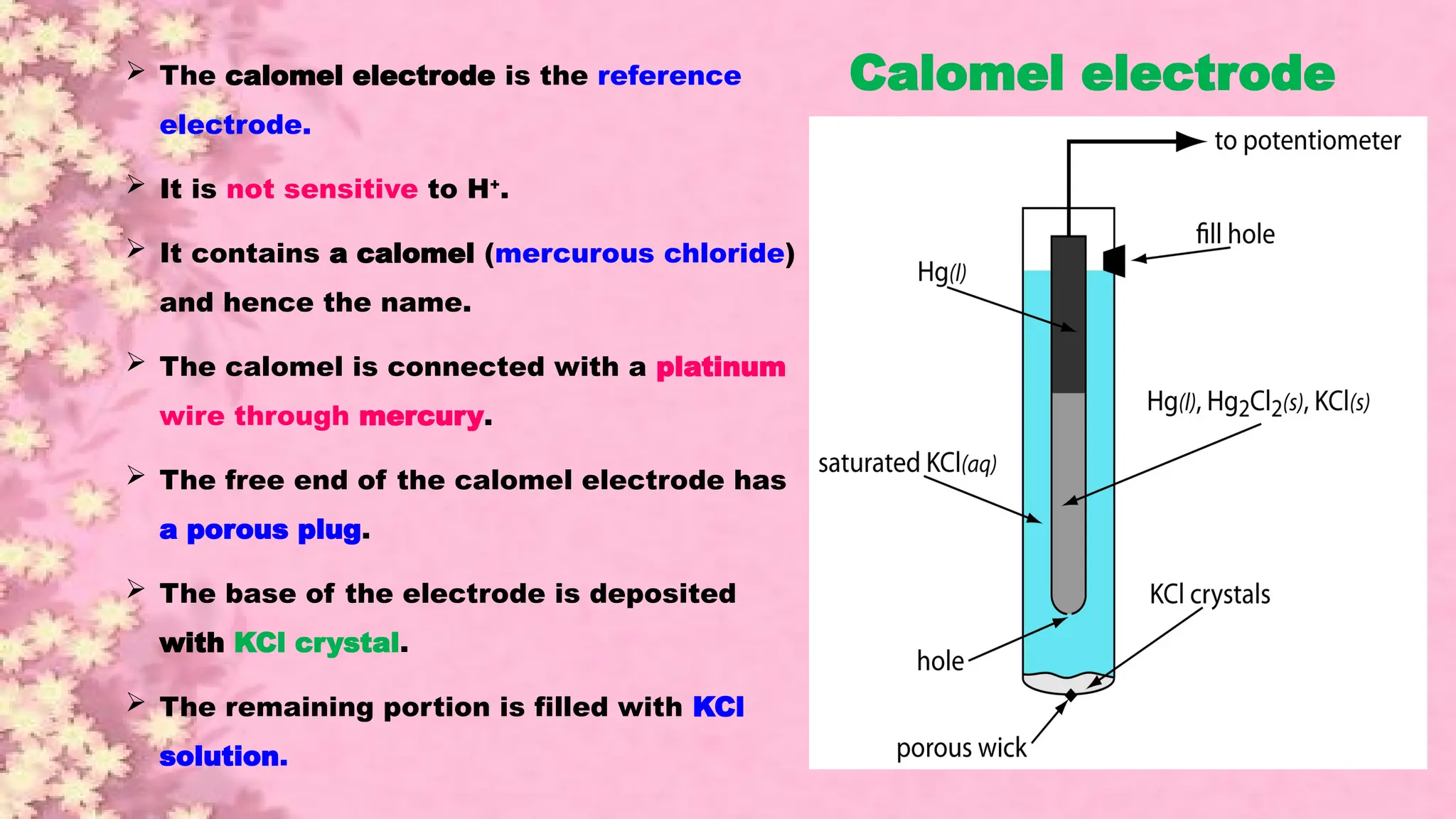
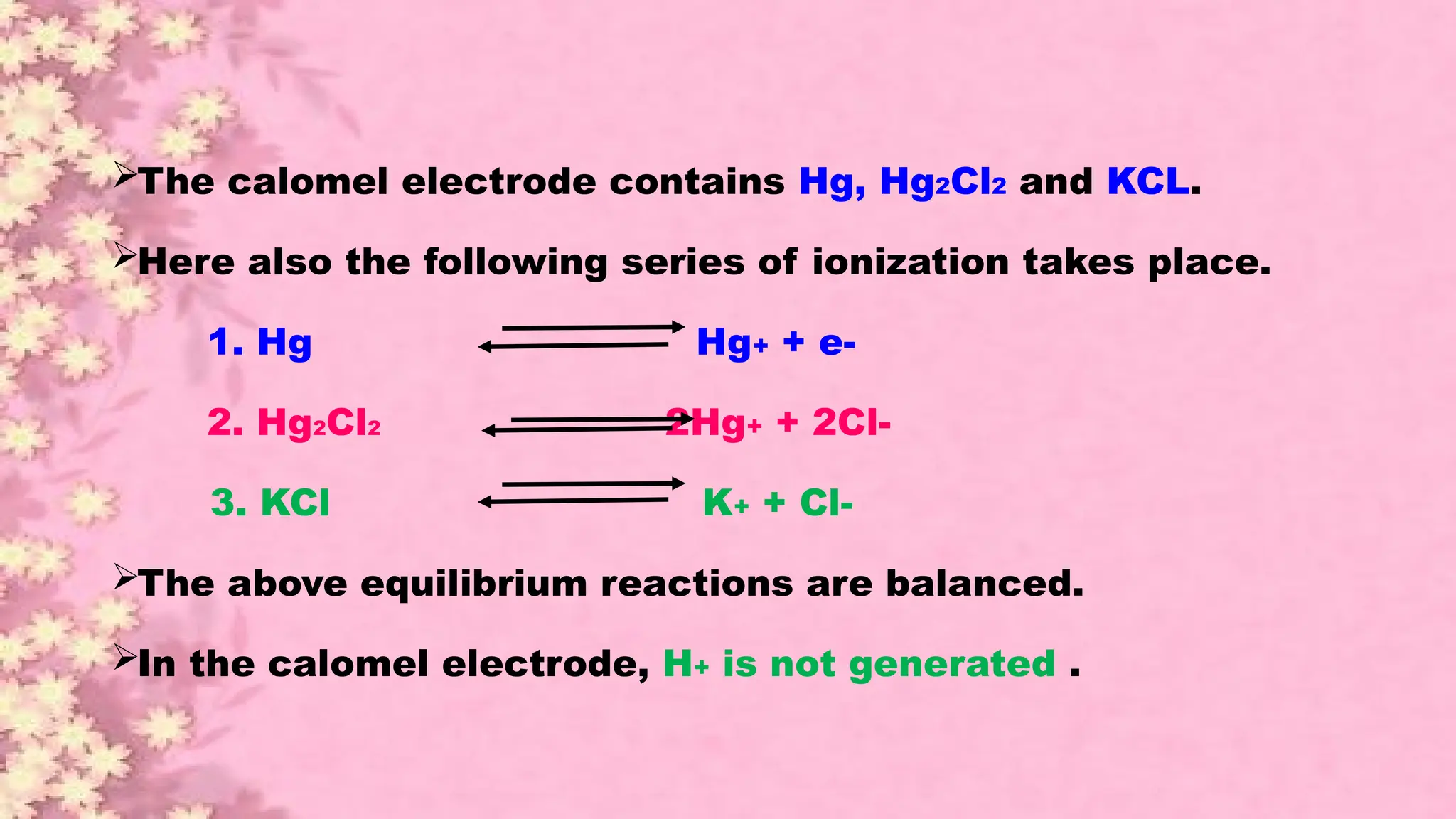
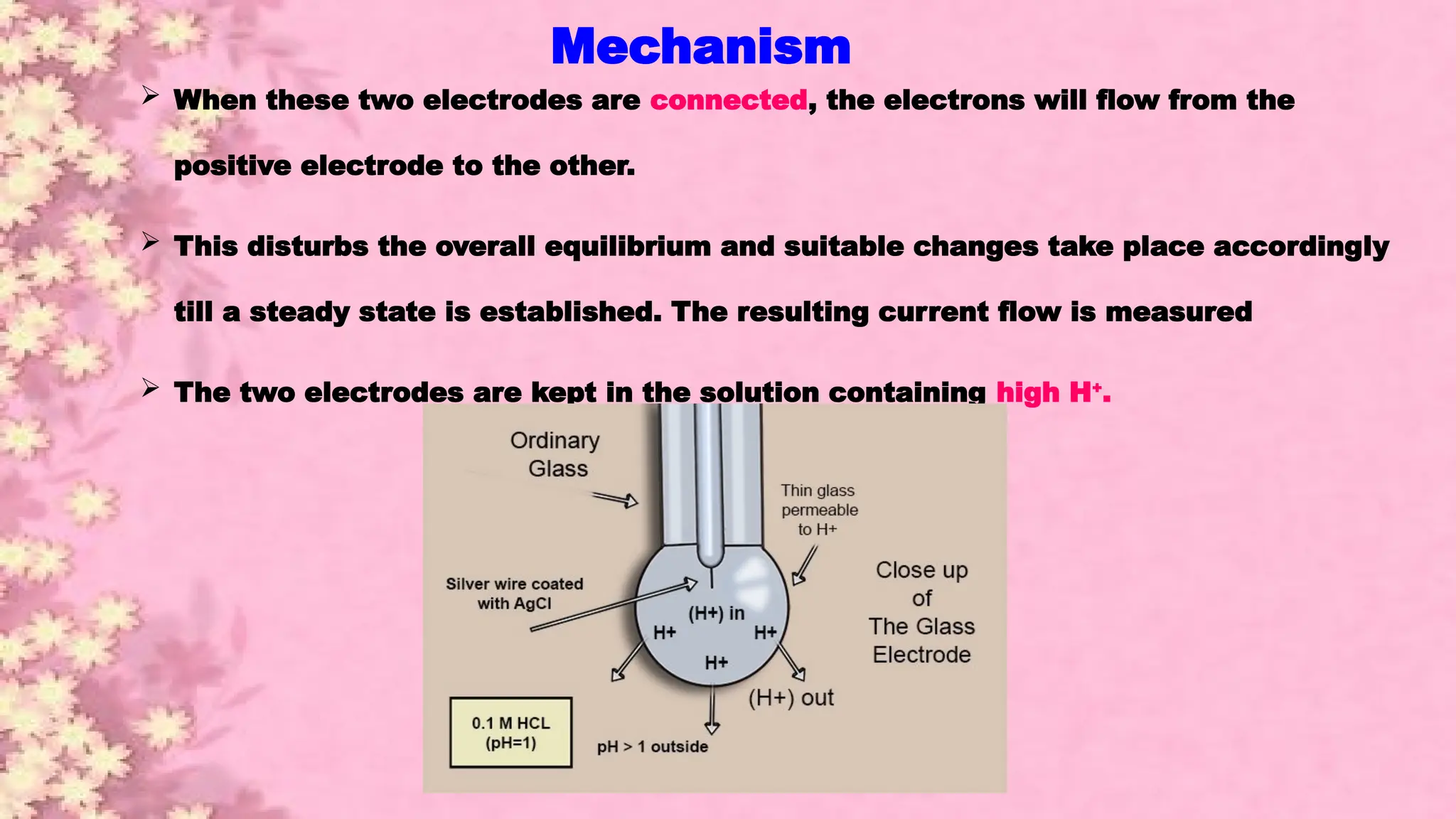
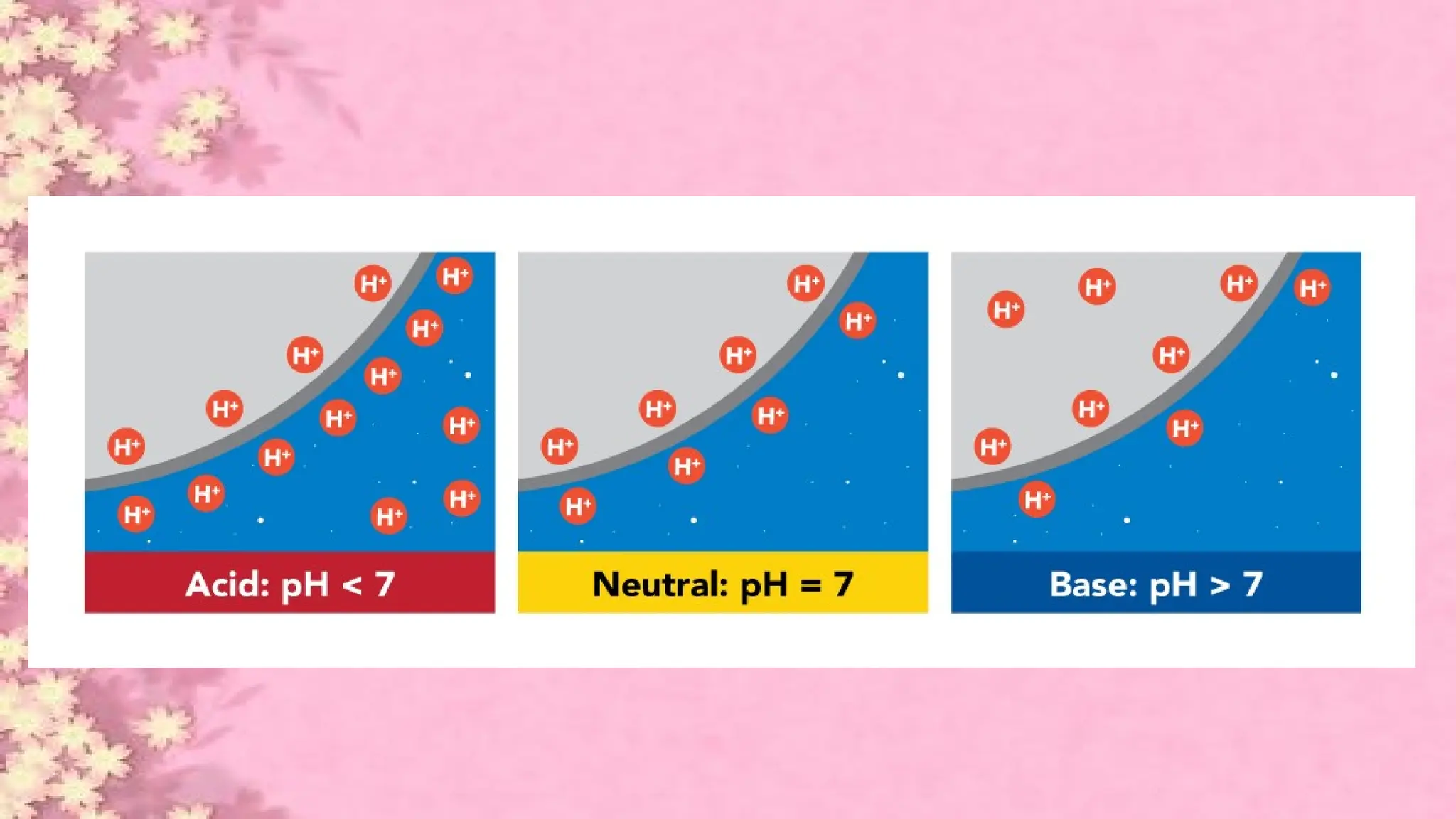
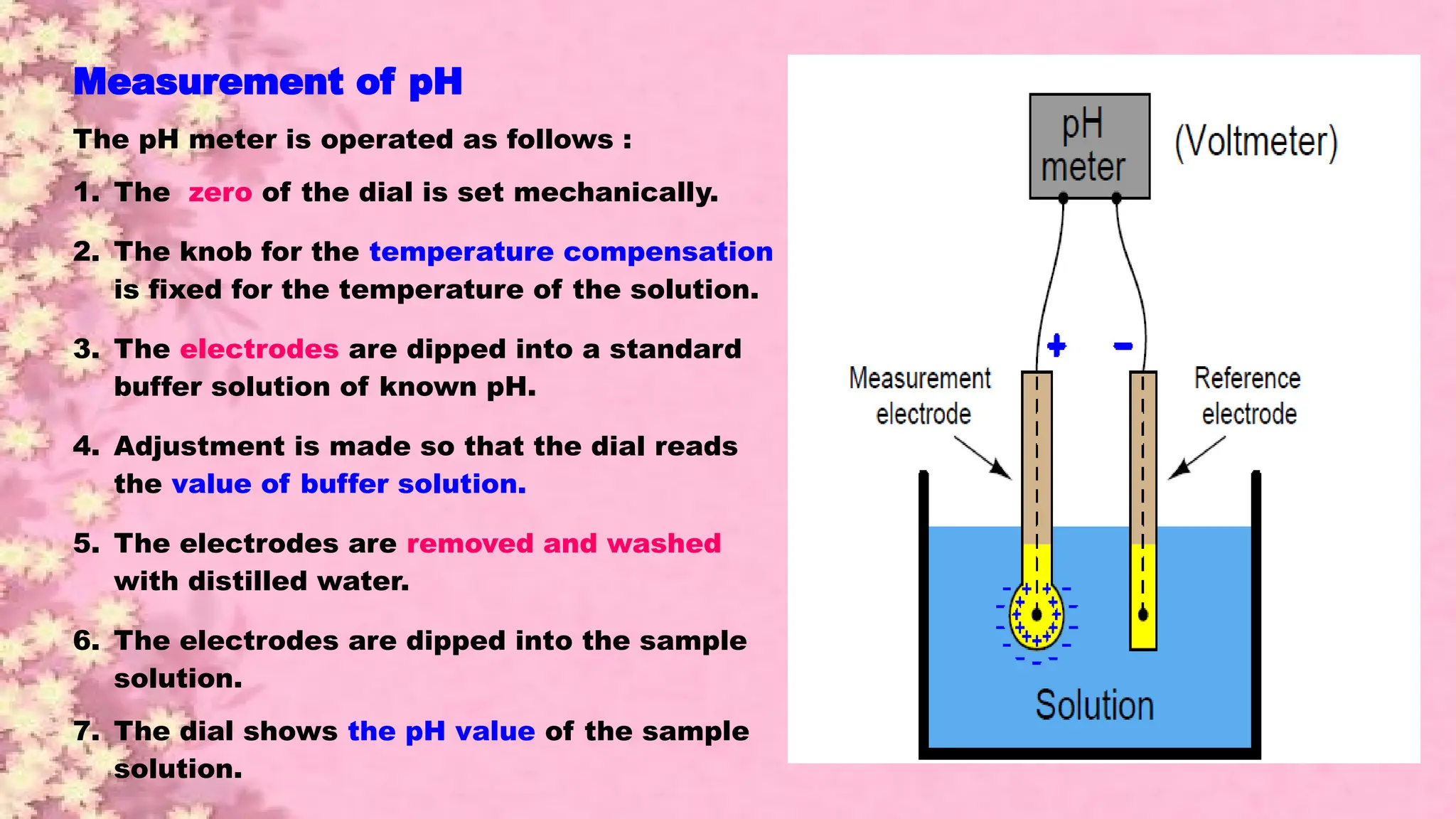
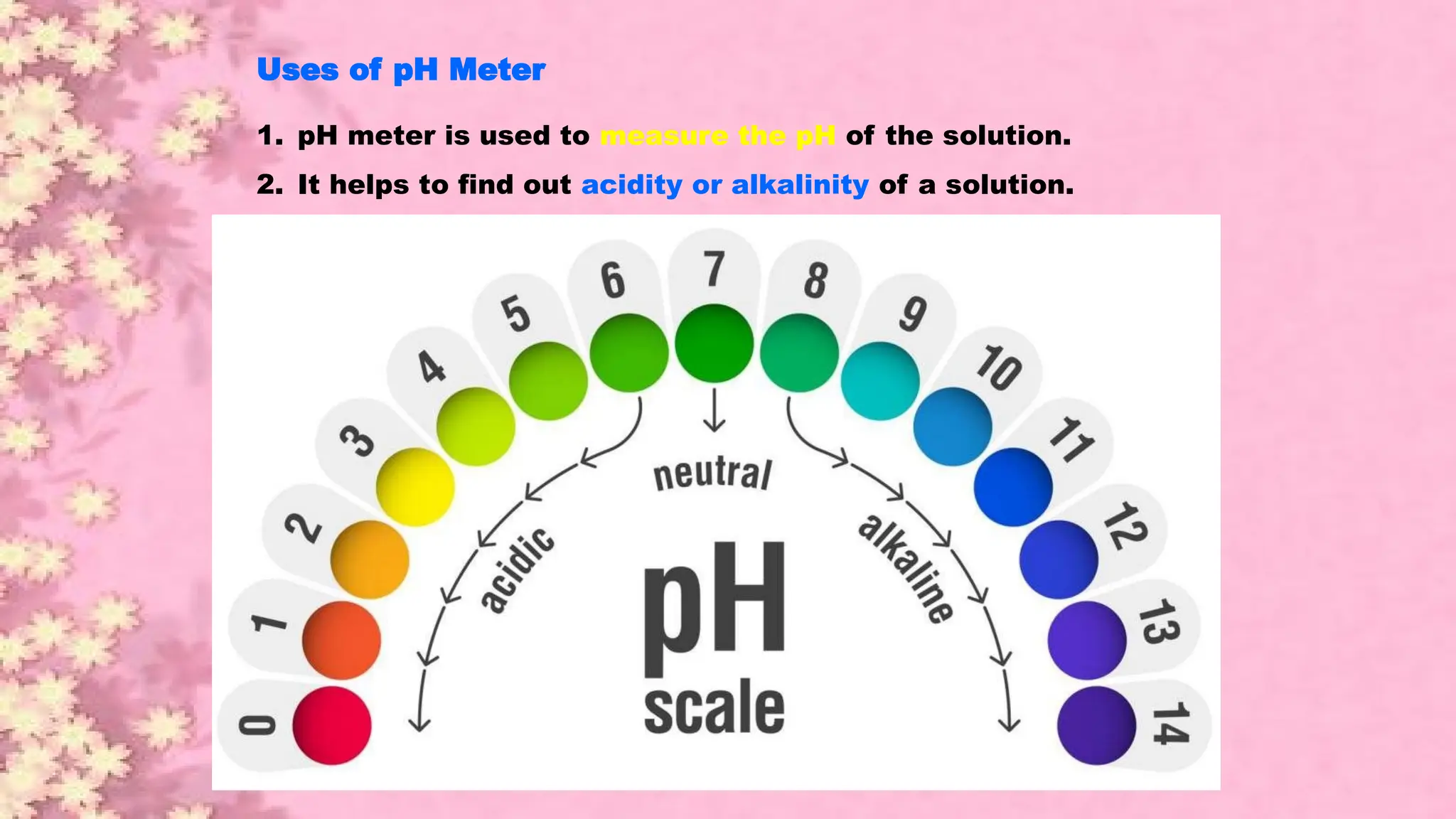
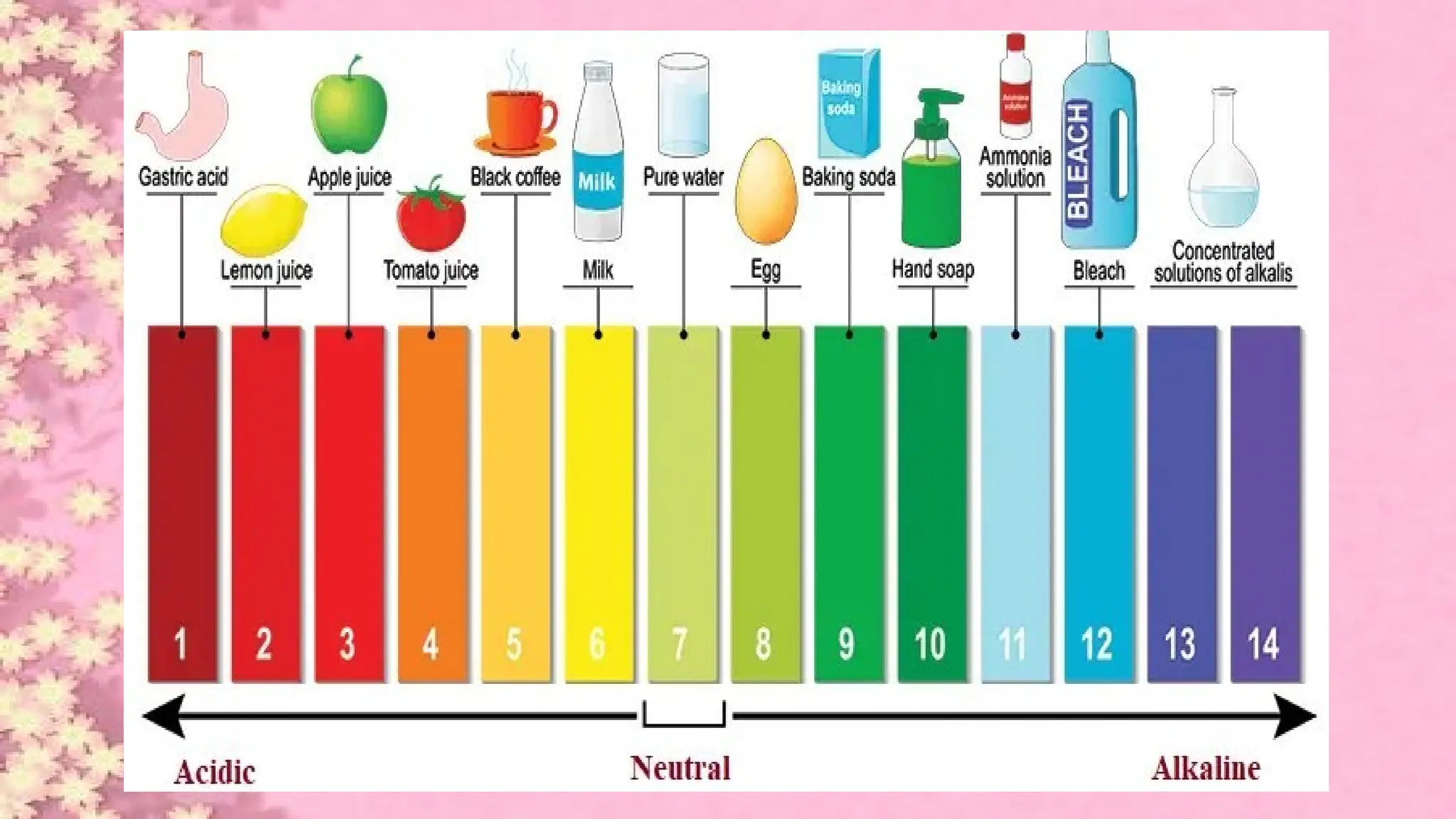
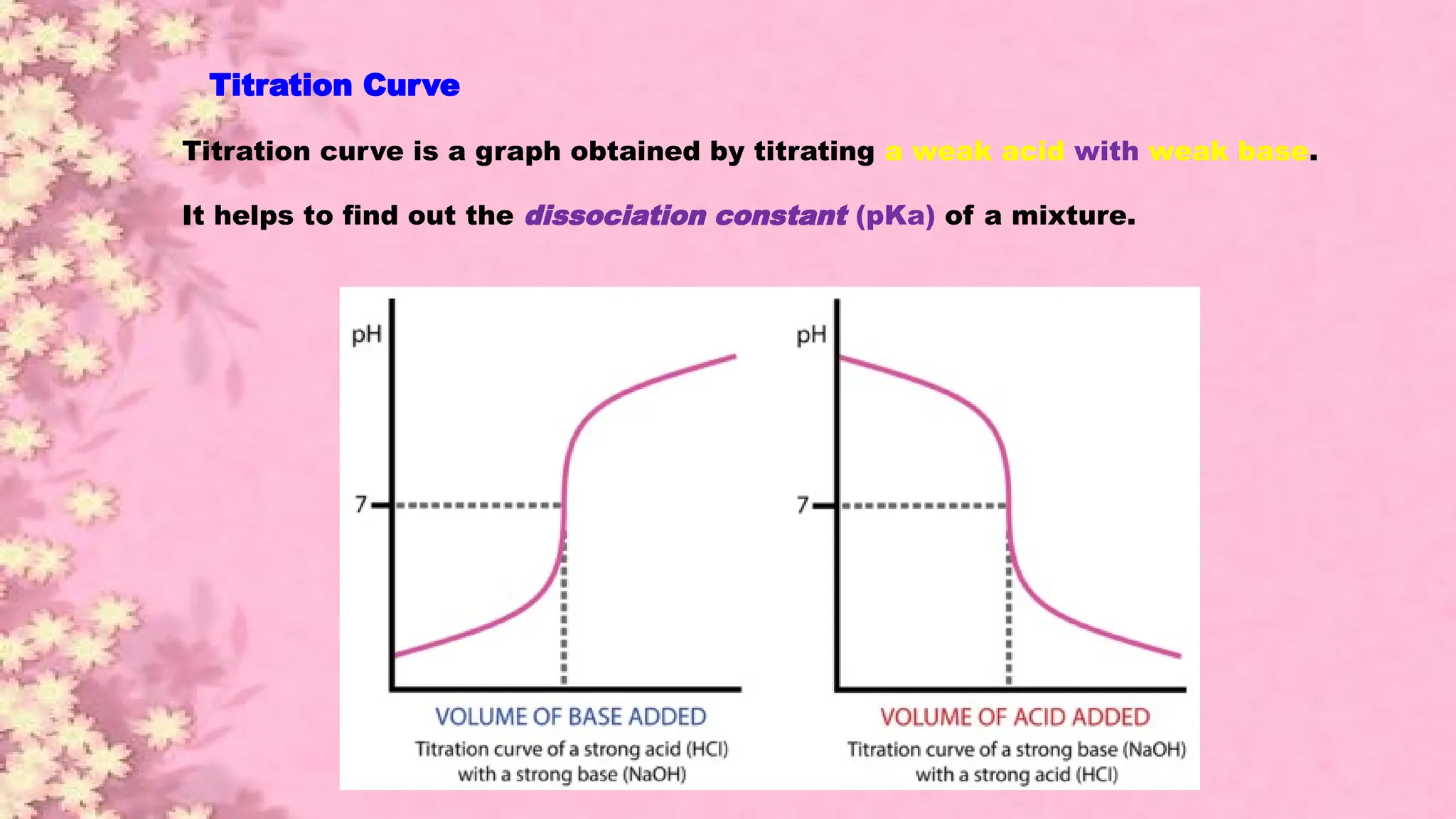
A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH.
The pH meter is an electronic instrument used to measure the pH of a solution.
The pH meter is of two types, namely digital pH meter and manual pH meter.