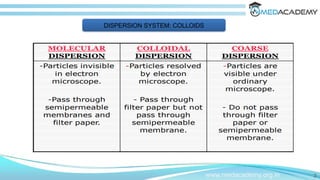
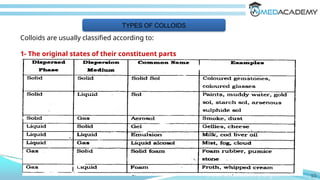
The document discusses the characteristics and classifications of colloids, detailing their structure as composite systems of dispersed and dispersion phases. It explores the factors affecting colloidal behavior, such as particle size, shape, and interaction with the dispersion medium, alongside optical and kinetic properties like Brownian motion and sedimentation. Additionally, it describes the types of colloidal systems, including lyophilic, lyophobic, and association colloids, as well as methods for determining properties such as molecular weight and viscosity.