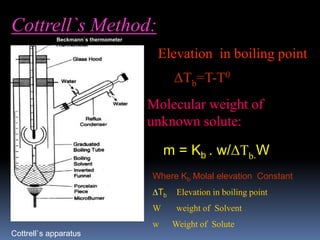
The document provides an overview of colligative properties of dilute solutions, including boiling point elevation and freezing point depression, and describes methods for determining the molecular weight of solutes using these properties. It discusses equations for calculating changes in boiling and freezing points, examples, and numerical problems related to the topic. Additionally, it mentions the Van 't Hoff factor and references for further reading on physical chemistry.