This document discusses different definitions of acids and bases that were proposed over time:
1. Lavoisier defined acids as containing oxygen. Liebig later defined acids as containing hydrogen that can be replaced by a metal.
2. Arrhenius defined acids as substances that produce hydrogen ions (H+) in aqueous solution and bases as substances that produce hydroxide (OH-) ions.
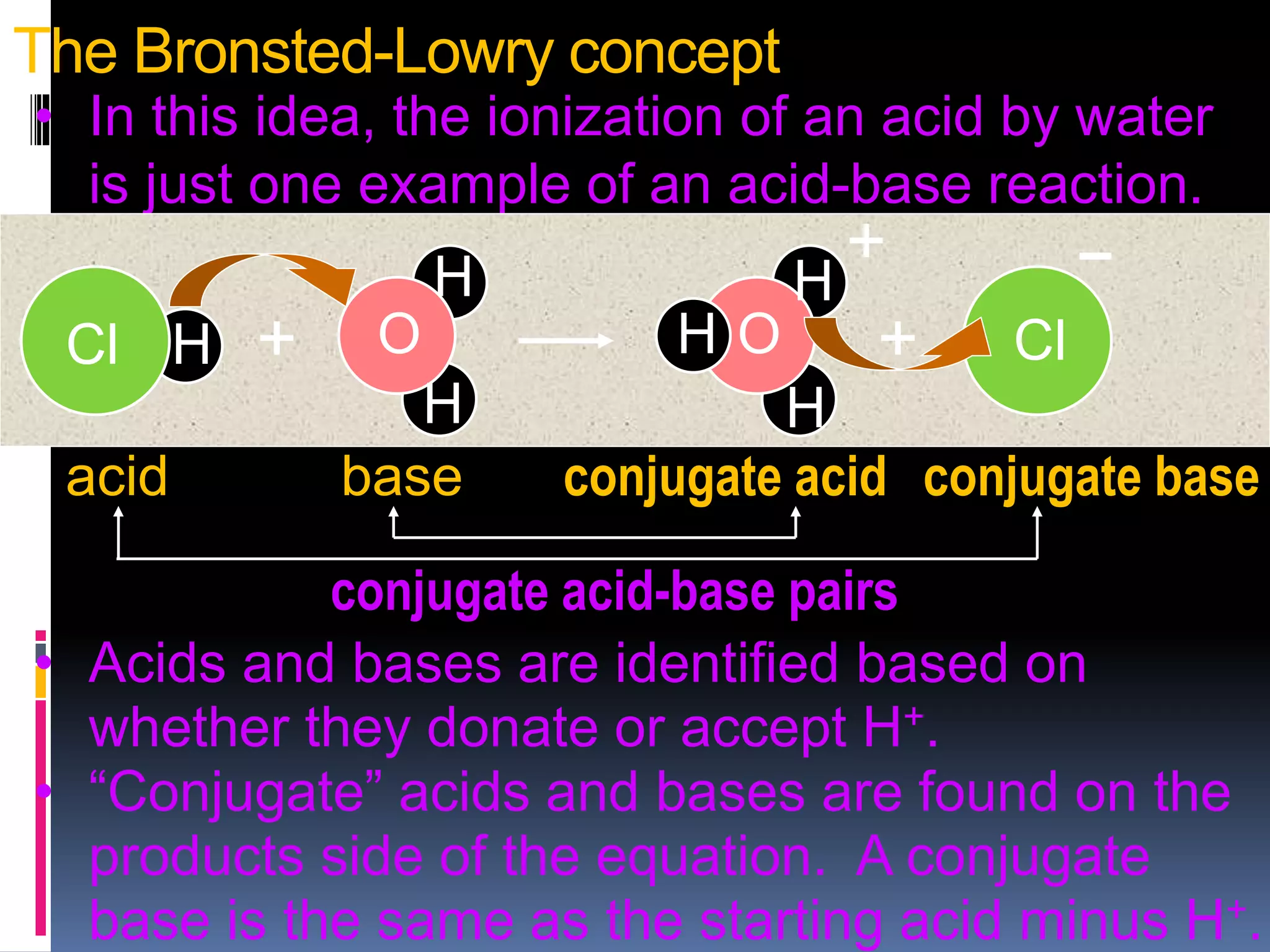
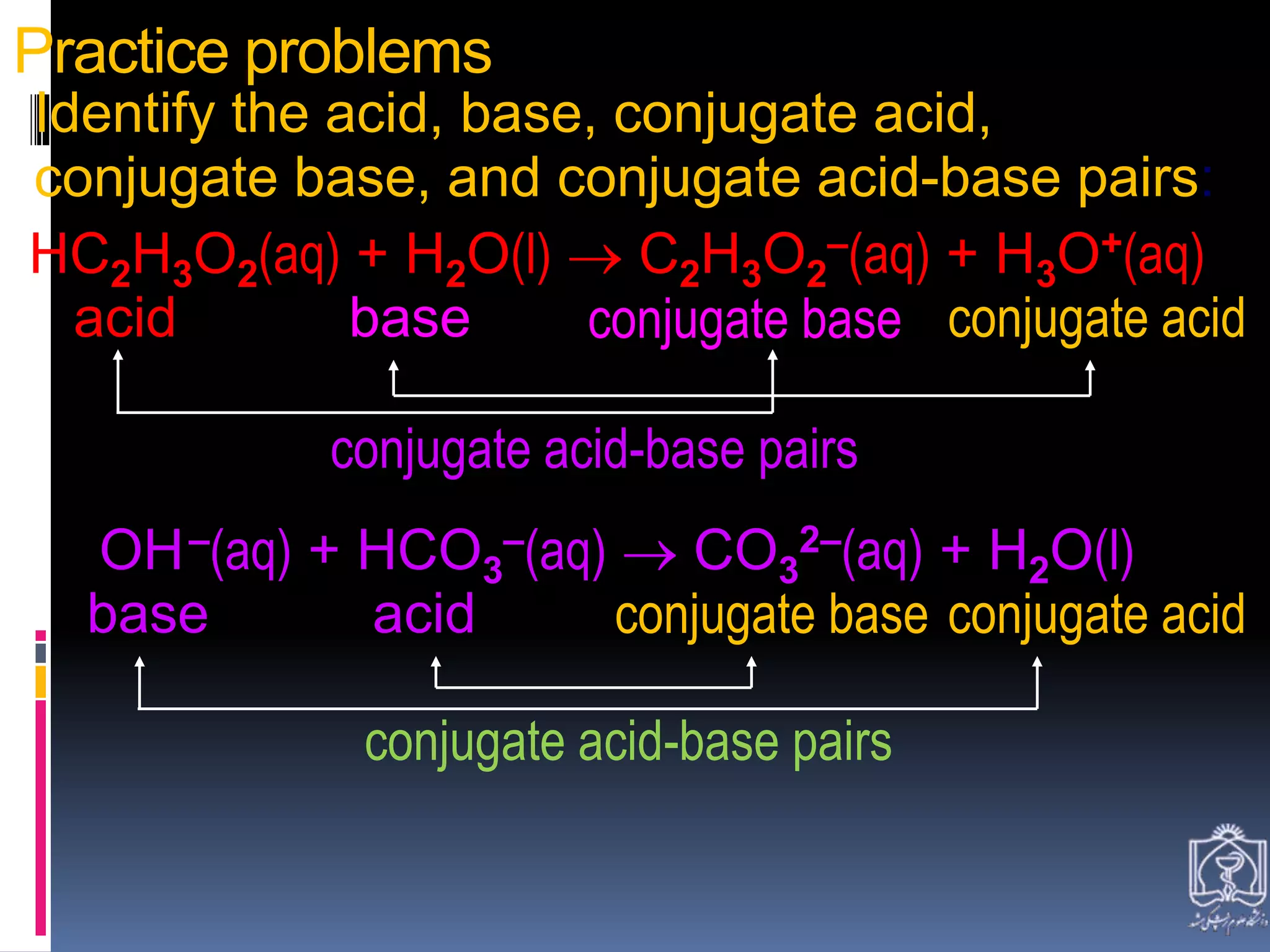
3. Brønsted and Lowry defined acids as proton donors and bases as proton acceptors in acid-base reactions, forming conjugate acid-base pairs.
4. Lewis expanded the definition to include electron pair donors and acceptors in both aqueous and non-aqueous reactions.
It also discusses














![In differentiation from the Arrhenius definition, the Brønsted-Lowry
definition postulates that for each acid, there is a conjugate acid and base
or "conjugate acid-base pair" that is formed through a complete reaction,
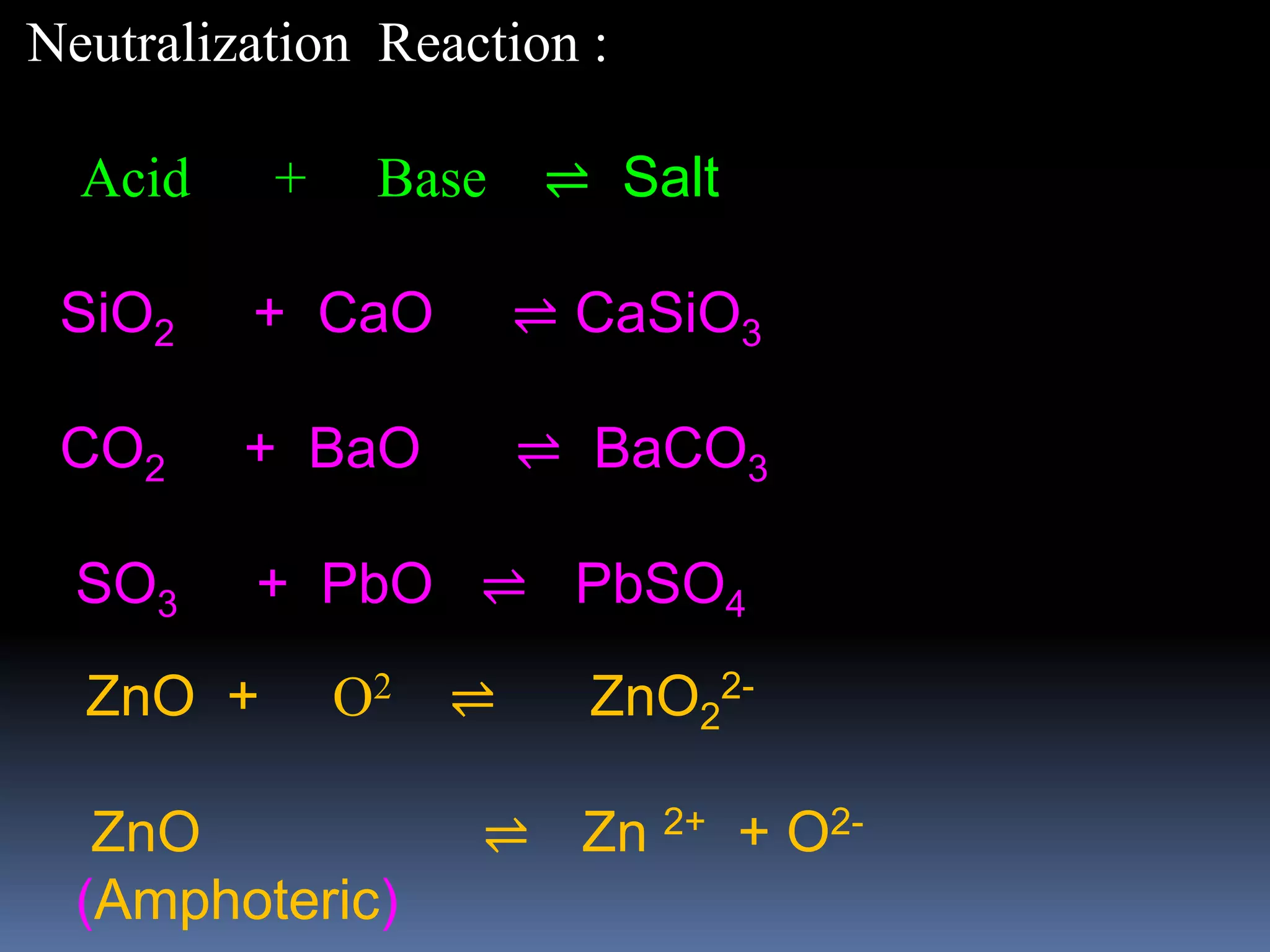
which also includes water, which is amphoteric
For example, zinc oxide (ZnO) reacts differently depending on the pH of the solution:
In acids: ZnO + 2H+ → Zn2+ + H2O
In bases: ZnO + H2O + 2OH- → [Zn(OH)4]2-
Base (Proton Acceptor): H2O + HCl → H3O+ + Cl−
Acid (Proton Donor): H2O + NH3 → NH4
+ + OH−
(Indeed, it can do both at once: 2H2O → H3O+ + OH−)](https://image.slidesharecdn.com/acid-basetheorynhb2020-200521035507/75/ACID-BASE-THEORY-17-2048.jpg)






![The Lewis definition of acid base reactions, devised by Gilbert N. Lewis
in 1923 is an encompassing theory to the Brønsted-Lowry and solvent-
system definitions with regards to the premise of a donation
mechanism, which conversely attributes the donation of electron
pairs from bases and the acceptance by acids, rather than protons
or other bonded substances and spans both aqueous and non-aqueous
reactions
Ag+ + 2 :NH3 → [H3N:Ag:NH3]+
A silver cation reacts as an acid with ammonia which acts as
an electron-pair donor, forming an ammonia-silver adduct
Acid - An electron
pair acceptor
Base - An electron
pair donor](https://image.slidesharecdn.com/acid-basetheorynhb2020-200521035507/75/ACID-BASE-THEORY-26-2048.jpg)