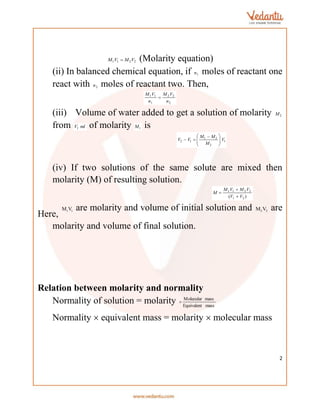
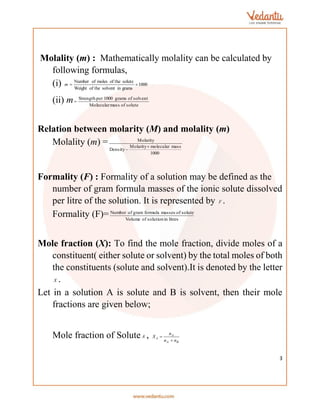
This document provides an overview of various methods for calculating the concentration and properties of solutions, including parts per million (ppm), normality, molarity, molality, formality, and mole fraction. It details the relationships between these measurements, alongside concepts like osmotic pressure, boiling point elevation, and freezing point depression. Additionally, it introduces Van't Hoff's factor for understanding solute behavior in solutions.