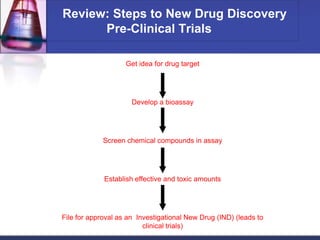
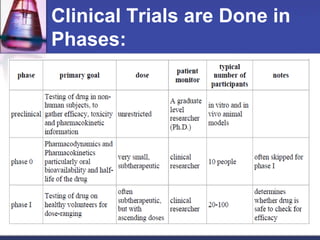
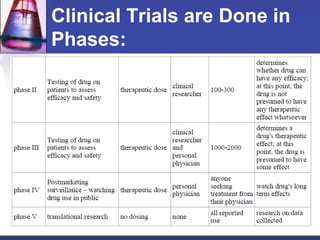
Pre-clinical and clinical trials are processes used to test drugs and medical devices for safety and efficacy before human use. Pre-clinical trials involve basic research and testing in animals. If promising, an Investigational New Drug application is filed with the FDA to begin clinical trials with humans. Clinical trials are conducted in phases, starting with small safety trials and progressing to large efficacy trials. If successful, final approval is sought from the FDA through a New Drug Application. The entire drug development process from basic research to approval typically takes 12-15 years and costs $100-800 million per drug.