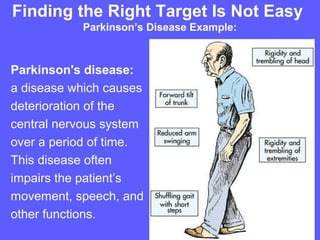
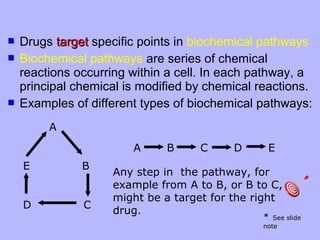
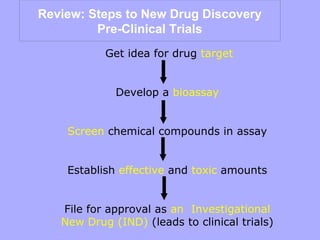
The document describes the process of pre-clinical trials for testing new drugs. It involves:
1) Identifying a drug target through basic research and applying that knowledge through applied research.
2) Developing a bioassay, usually using animal or cell models, to test the drug's safety and effectiveness.
3) Establishing the effective and toxic doses of the drug through screening in the bioassay system.
4) Filing for approval as an Investigational New Drug with the FDA if results show promise, allowing tests in human clinical trials. The goal of pre-clinical trials is to determine if a new drug or medical device is safe and effective before testing in humans using the scientific method.