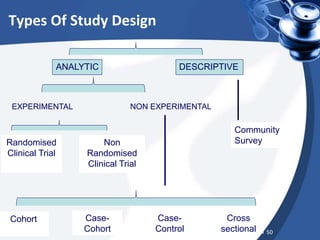
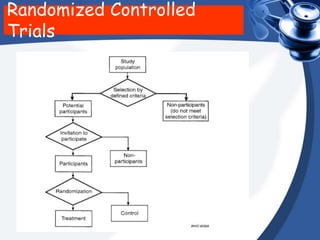
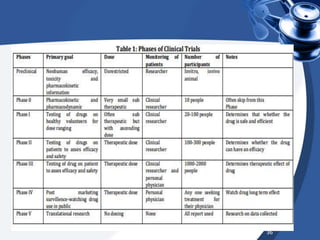
Clinical trials are conducted in phases to evaluate the safety and efficacy of new medical interventions in humans. Phase I trials involve small groups of subjects and focus on safety, tolerability and dosing of new drugs. Phase II trials involve larger groups of patients and aim to establish effectiveness and optimal dosing. Phase III trials involve thousands of subjects and compare the new medical intervention to the current standard of care or placebo under blinded conditions. The results of phase III trials are required for regulatory approval prior to widespread use and administration of the new medical intervention.







































![● No adverse effects observed, the dose is
escalated, and a new group of subjects are
then given a higher dose than previous
group
● This is continued until intolerable side
effects or adverse effects start showing up
[at which point the drug is said to have
reached the Maximum tolerated dose
(MTD)].](https://image.slidesharecdn.com/clinicaltrialsdhruva-150412112241-conversion-gate01/85/Clinical-trials-dhruva-41-320.jpg)






![EARLY PHASE II
[Establish Dose range]
● Usually a Randomised Single Blind Design
● A small number of patients upto 200
● Potential therapeutic benefits, side effects
and adverse effects are observed
● Main obejective – to estabilish a dose range
for more definitive trials to be undertaken in
the next late phase here](https://image.slidesharecdn.com/clinicaltrialsdhruva-150412112241-conversion-gate01/85/Clinical-trials-dhruva-48-320.jpg)
![Late Phase II
[Efficacy]
● Randomized Double Blind Design
● Larger number of patients: 200-400
● Specifically designed to study efficacy (how
well the drug wroks at prescribed doses)](https://image.slidesharecdn.com/clinicaltrialsdhruva-150412112241-conversion-gate01/85/Clinical-trials-dhruva-49-320.jpg)