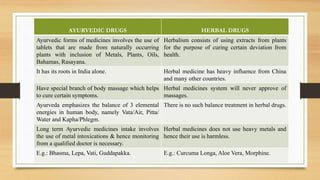
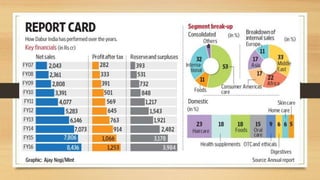
The document discusses herbal drug regulation in India. It provides an overview of the types of herbal formulations regulated in India, the advantages of herbal drugs, market statistics, and the various schedules and rules that govern herbal drugs under the Drugs and Cosmetics Act. It also discusses Ayush regulations for herbal drugs, requirements for clinical trials, and issues related to promoting herbal drugs in India. The future prospects section outlines opportunities to improve regulation and development of the herbal drug industry in India.



































![Comparison with regulations
of EUROPE
Most Stringent
For marketing authorization------bibliographic evidence & preclinical
safety data. As per traditional herbal medicinal product directive
(2004/24/EC).
For registration------ Quantitative and Qualitative Documents as per
[ Article 8 (3) (a) to , (h) (j)(k) ] & [Article 11 (4) of Directive
2001/83/EC] a summary of product characteristics.
Expensive for Indian Herbal Manufacturer](https://image.slidesharecdn.com/herbalregulations-180405173002/85/Herbal-regulations-39-320.jpg)