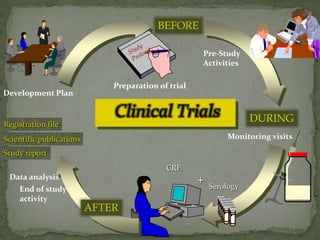
This document discusses clinical trials, including their objectives, key aspects, and participating parties. The main points are:
Clinical trials examine and evaluate the safety and efficacy of therapies in human subjects. Their objectives include developing protocols, screening and enrolling participants, monitoring safety, and generating reports. Key elements are experimental units (subjects), treatments being tested, and ways of evaluating outcomes. Clinical trials involve various parties like sponsors, investigators and their teams, reviewing ethics boards, institutions hosting the trials, and regulatory authorities. They progress through defined pre-clinical and phases I-IV to develop therapies and ensure subjects' protection.












![ Healthy volunteers : Uniformity of subjects:
age, sex, nutritional status [Informed consent
a must]
Exception: Patients only for toxic drugs
eg:- Anti HIV, Anticancer
– Exclusion criteria
Women of child bearing age, children,](https://image.slidesharecdn.com/clinicaltrial-170212164014/85/Clinical-trial-14-320.jpg)
![ First in patient [ different from healthy volunteer]
Early phase [20 – 200 patients with relevant disease]
Therapeutic benefits & ADRs evaluated
Establish a dose range to be used in late phase
Single blind [Only patient knows] comparison with
standard drug
Late phase [ 50 – 500]
Double blind
Compared with a placebo or standard drug](https://image.slidesharecdn.com/clinicaltrial-170212164014/85/Clinical-trial-15-320.jpg)
![ Assesses efficacy against a defined therapeutic
endpoint
Detailed Pharmacokinetic & Pharmacodynamic
data
Establishes a dose & a dosage form for future
trials
Takes 6 months to 2 years [ 35% success rate]](https://image.slidesharecdn.com/clinicaltrial-170212164014/85/Clinical-trial-16-320.jpg)
![ Large scale, Randomised, Controlled trials
Target population: 250 – 1000 patients
Performed by Clinicians in the hospital
Minimises errors of phases I and II
Methods
Multicentric Ensures geographic & ethnic variations
Diff patient subgroups Eg pediatric, geriatric, renal impaired
Randomised allocation of test drug /placebo / standard drug
Double blinded:
Cross over design
Vigilant recording of all adverse drug reactions
Rigorous statistical evaluation of all clinical data
Takes a long time: up to 5 years [25% success]](https://image.slidesharecdn.com/clinicaltrial-170212164014/85/Clinical-trial-17-320.jpg)

![ No fixed duration / patient population
Starts immediately after marketing
Report all ADRs
Helps to detect
rare ADRs
Drug interactions
Also new uses for drugs [Sometimes called Phase V]](https://image.slidesharecdn.com/clinicaltrial-170212164014/85/Clinical-trial-19-320.jpg)

![1. Patient / Healthy volunteer
2. Clinical Pharmacologist, Clinical
Investigator & team: [Qualified and
competent]
3. Institution where trials are held :
[Approval required]
4. Ethical Review Board or Institutional
Ethical Committee:
5. Sponsor
6. Regulatory Authorities](https://image.slidesharecdn.com/clinicaltrial-170212164014/85/Clinical-trial-21-320.jpg)
![ Patient / Healthy volunteer : Subject of
the trial
Clinical Pharmacologist, Clinical
Investigator & team:
– Conducts the clinical trial; reports all
adverse events
–
Institution where trials are held :
– Provides all facilities [Approval required]](https://image.slidesharecdn.com/clinicaltrial-170212164014/85/Clinical-trial-22-320.jpg)
