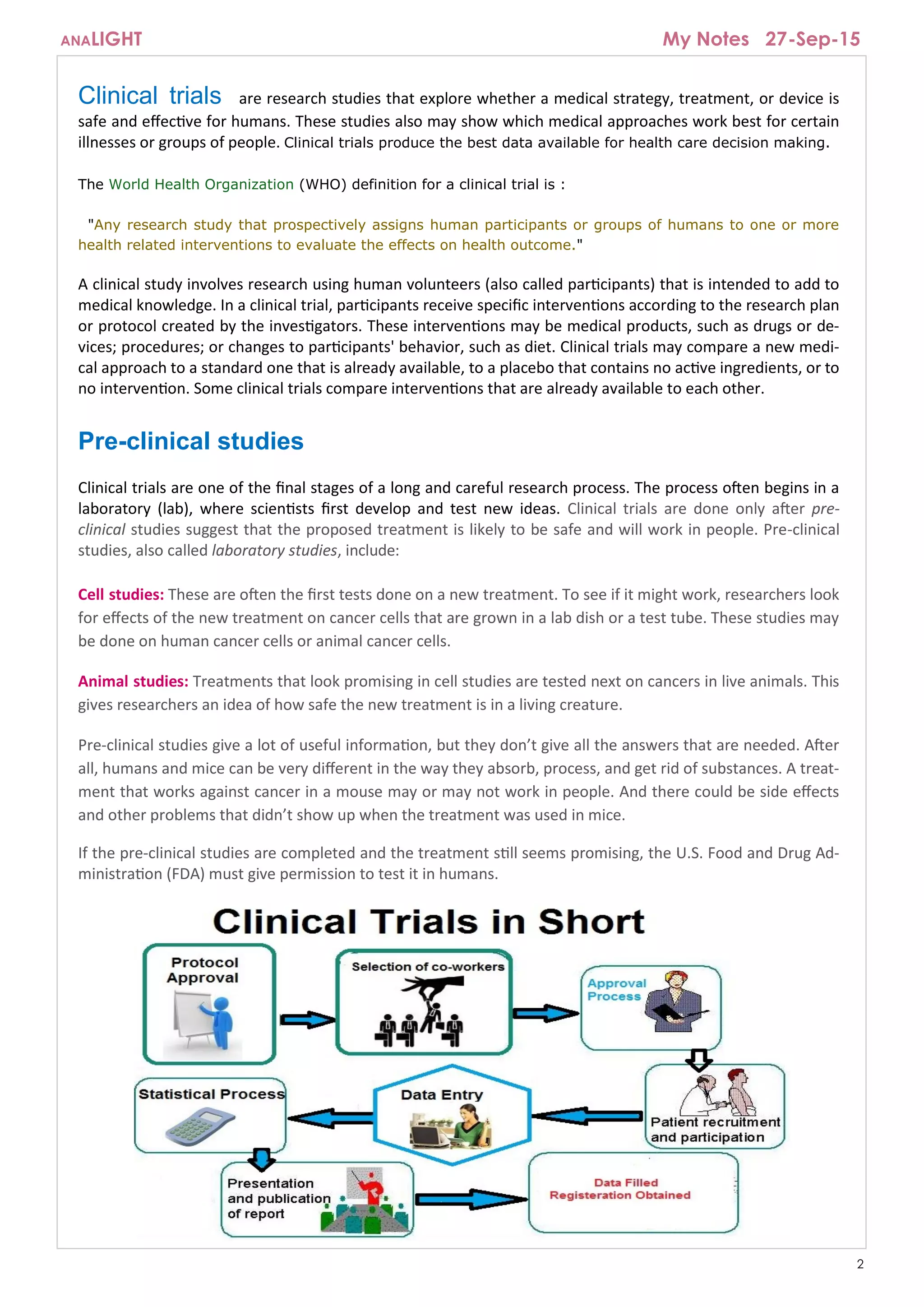
The document provides an overview of clinical trials, which are research studies to evaluate the safety and effectiveness of medical treatments or devices in humans. It explains the process of conducting clinical trials, including the phases from pre-clinical studies to post-marketing evaluations, and emphasizes the importance of evidence from these trials for healthcare decision-making. The document also discusses eligibility criteria for participants, the role of sponsors, and the expectations during a clinical trial.