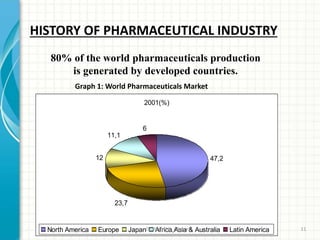
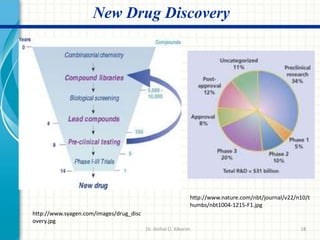
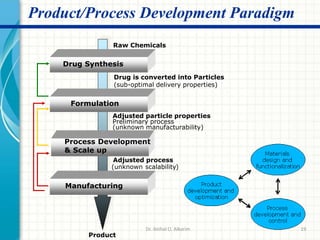
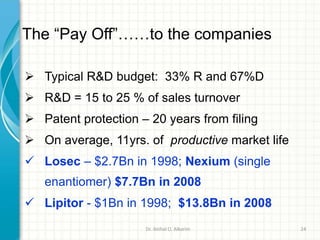
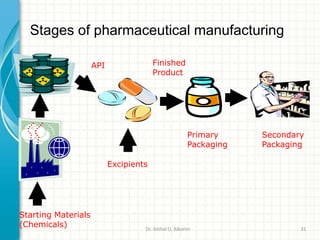
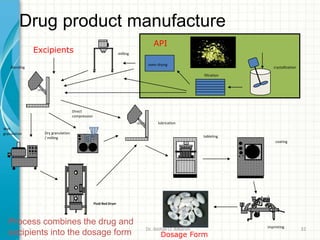
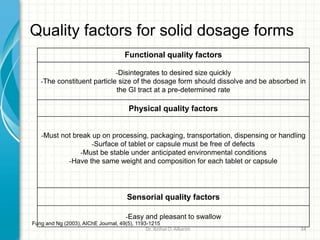
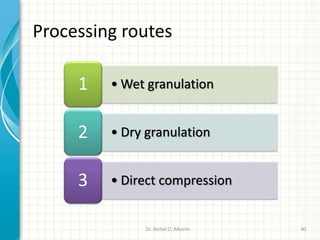
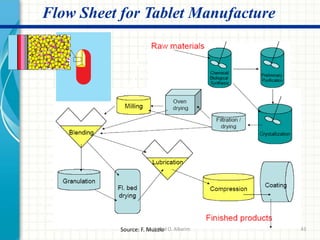
Pharmaceutical manufacturing involves the industrial scale synthesis and processing of drug products. Key steps include active pharmaceutical ingredient (API) synthesis, combining APIs and excipients, and processing the mixture into solid oral dosage forms like tablets through unit operations such as milling, blending, granulation, drying, compression, and coating. Quality is ensured through strict adherence to good manufacturing practices (GMP) regulations. Excipients are other ingredients included in drug products that aid processing and delivery of the active drug. Common processing routes for solid oral dosage forms are direct compression, dry granulation, and wet granulation which involve different sequences of unit operations.