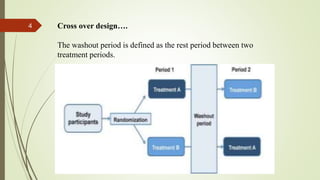
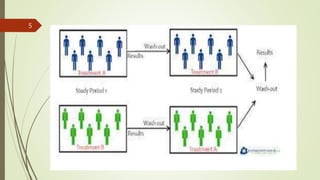
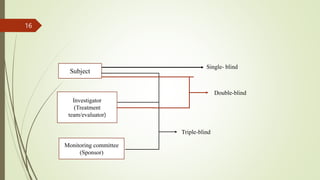
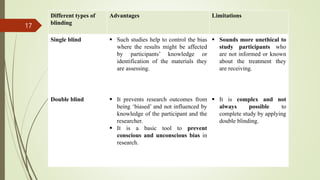
The document provides an overview of crossover design, placebo, and blinding techniques in clinical trials, highlighting the advantages and disadvantages of crossover designs, such as within-patient comparisons and potential carry-over effects. It describes different types of placebos and blinding methods, including single, double, and triple blinding, each with their own advantages, limitations, and relevance in reducing bias. Overall, the content focuses on the methodologies used to enhance the reliability and validity of clinical research outcomes.