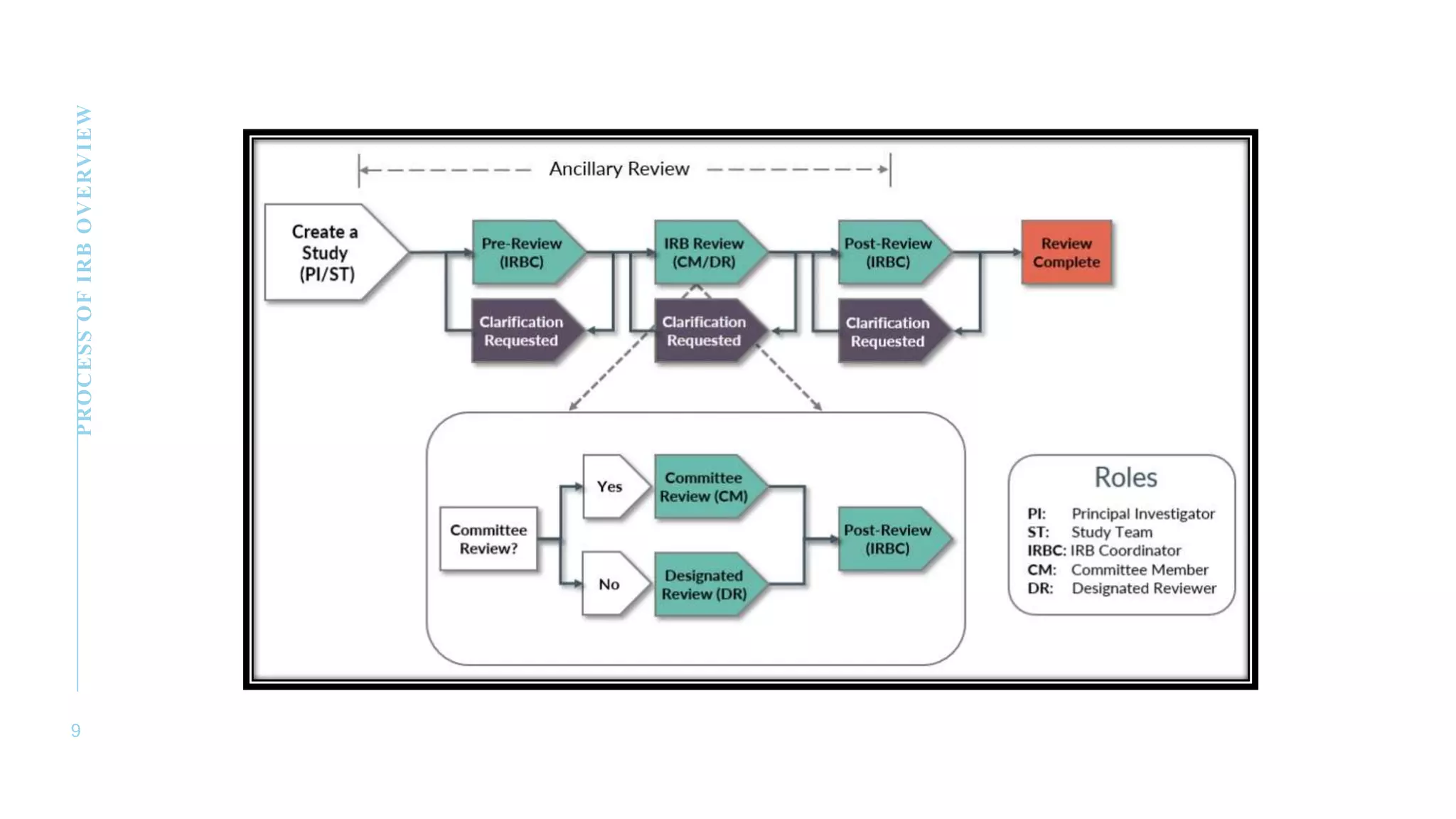
The document discusses the role and responsibilities of an Institutional Review Board/Independent Ethics Committee (IRB/IEC). It states that an IRB/IEC reviews clinical trial protocols to ensure the ethical treatment of study participants and protection of their rights and well-being. The IRB/IEC is composed of at least five members with diverse qualifications and one member from a non-scientific discipline. It is responsible for approving, monitoring and reviewing research involving humans. The IRB/IEC conducts initial and annual reviews of trial procedures and documentation. All records are maintained for at least three years.