1) Clinical trials involve prospectively assigning human participants to health interventions to evaluate effects on outcomes. They aim to carefully and ethically answer precisely framed questions.
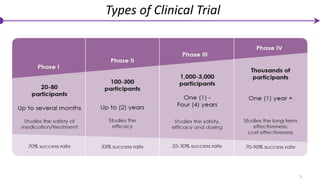
2) Clinical trials are classified into phases based on goals, with Phase 0 trials involving small doses and numbers of participants to assess safety.
3) Randomization, blinding, inclusion/exclusion criteria, and sample size are important design considerations to reduce bias and ensure results reflect the interventions rather than other factors. Statistical analysis then determines if any effects seen are real or due to chance.



![The design specification should be able to reflect the:
• type of treatment and number of treatments
• method of randomization
• type of blinding
• type of study question
• study medication
During Protocol Development
5
Bakhai et al, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-5-320.jpg)
![General considerations
Source of patients
Phase I and II pharmaceutical studies usually involve small numbers of patients and
are therefore frequently performed in specialist centers (e.g., asthma laboratories in
hospitals with dedicated research units). – ‘Center of excellence’.
Investigators and centers
Investigators must have an understanding of what is required for the trial so that
they can clearly communicate this information to potential participants.
Enrollment of more centers, not economical, given the resources needed to train
new centers.
6
Bakhai et al, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-6-320.jpg)



![Randomization
Randomization is the unpredictable allocation of a patient to a particular treatment
strategy in a clinical trial.
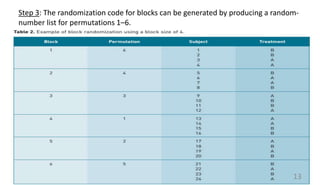
Common types of randomization methods are:
• simple randomization
• block randomization
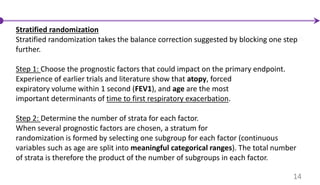
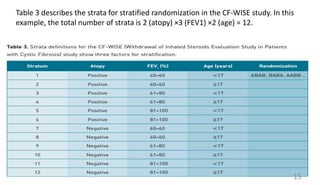
• stratified randomization
Simple randomization:
Tossing an unbiased coin, e.g., heads for treatment A and tails for treatment B. When
the next subject is to be assigned, previous allocations are not considered.
10
Bakhai et al, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-10-320.jpg)


![Blinding
Randomization can minimize bias, but it can still occur, however, if study personnel and
patients know the identity of the treatment, due to preconceptions and subjective
judgment in reporting, evaluation, data processing, and statistical analysis.
With respect to blinding, there are four general types of blinded studies in clinical trials
:
• open/unblinded
• single blinded
• double blinded
• triple blinded
17
Clinicaltrials.gov, Bakhai et al, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-17-320.jpg)




![Ronald Fischer (1890-1962)
After a randomized controlled trial is conducted, statisticians help
determine whether any observed difference between outcomes
in the experimental and control arms are real, or simply chance
occurrences.
The process to which Fisher’s methods can be applied is called
hypothesis testing.
In hypothesis testing a null hypothesis (H0) is articulated which is
typically a statement of no difference between experimental and
control patient populations.
22
Perlmutter, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-22-320.jpg)
![Francis Bacon (1561-1626)
Evidence-based medicine depends on the
systematic accumulation of information about
how different treatments affect patients.
Using observations from a patient
sample to draw conclusions about its patient
population.
The scientific method is schematized in Figure
4 below.
“The Evolution”
24
Perlmutter, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-24-320.jpg)
![25
Perlmutter, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-25-320.jpg)

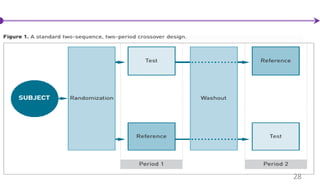
![Cross-Over Designs
• Subjects are randomized to sequences of treatments (A then B or B then A)
• Uses the patient as his/her own control
• Often a “wash-out” period (time between treatment periods) is used to avoid a
“carry over” effect (the effect of treatment in the first period affecting outcomes in
the second period)
• Can have a cross-over design with more than 2 periods
27
Bakhai et al, [2006], ICH E9](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-27-320.jpg)
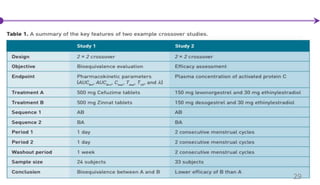



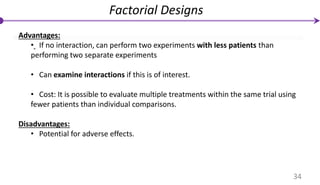
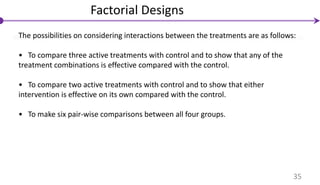
![Factorial Designs
Individuals are randomly assigned to two separate interventions (e.g.,
interventions A and B) and these interventions are each compared with their
corresponding control(s) .
• N/ 4 individuals are allocated to no treatment (control group).
• N/ 4 individuals are allocated to intervention A only.
• N/ 4 individuals are allocated to intervention B only.
• N/ 4 individuals are allocated to the combination of A + B simultaneously.
An important concept for these designs is interaction (sometimes called effect
modification)
Interaction: The effect of treatment A differs depending upon the presence or
absence of intervention B and vice-versa.
33
Bakhai et al, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-33-320.jpg)
![Equivalence Trials
A trial to compare the altered versus the original compound or drug to demonstrate
that there has been no loss of effectiveness or increase in side-effects.
36
Bakhai et al, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-36-320.jpg)


![Non Inferiority Trials
A non inferiority trial aims to demonstrate that the effect of a
new treatment is as good as, or better than, that of an active
comparator.
This is assessed by demonstrating that the new treatment is not worse than the
comparator by more than a specified margin (the non inferiority margin [δ]).
The following study will illustrate issues relating to the design and analysis of non
inferiority clinical trials.
39
Bakhai et al, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-39-320.jpg)

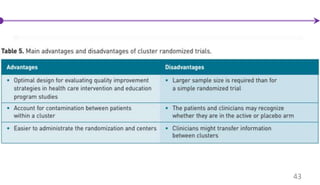
![Cluster Randomized Trials
Cluster randomized trials use a group of individuals, a hospital, or a community as
the unit of randomization.
41
Bakhai et al, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-41-320.jpg)

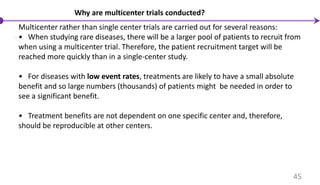
![Multicenter Trials
It allows a large number of patients to be recruited in a shorter time; the results are
more generalizable and contemporary to a broader population at large; and such
studies are critical in trials involving patients with rare presentations or diseases.
A multicenter trial is a trial that is performed simultaneously at many centers
following the same protocol. The activity at these centers is synchronized from a
single command center – the coordinating center.
44
Bakhai et al, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-44-320.jpg)

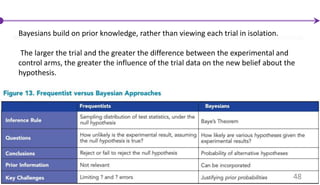
![Innovations in Trial Design
Thomas Bayes (1702-1762)
The Bayesian Concept
47
Perlmutter, [2006]](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-47-320.jpg)

![50
References
1. Bakhai, A., Wang, D., (2006), Clinical Trials: A Practical Guide to Design, Analysis
and Reporting, Remidica, London, UK, pp: 1-153.
2. ICH E9 guidelines.
3. Kalliomaki, J., Miller, F., Kagedal, M., Karlsten, R., (2012), Early phase drug
development for treatment of chronic pain—Options for clinical trial and program
design. Contemporary Clinical Trials, 33 (689-699), [Accessed on: 15 Nov 2013]
4. Lloyd, J., Raven, A., (1994), Handbook of Clinical Research, In: Lloyd, F., Clinical
Trial Design, 2nd Edition, Longman Group, UK Limited, pp: 92-113.](https://image.slidesharecdn.com/clinicaltrialdesign-160811082232/85/Clinical-trial-design-50-320.jpg)
