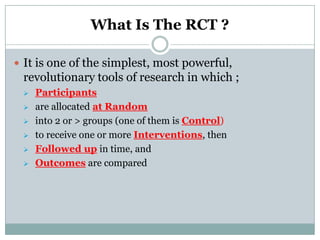
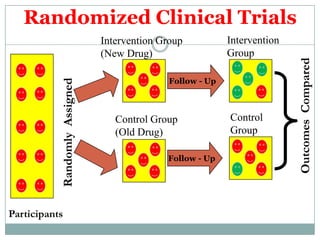
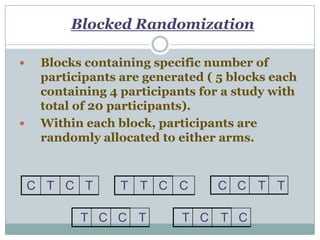
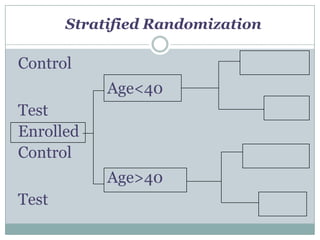
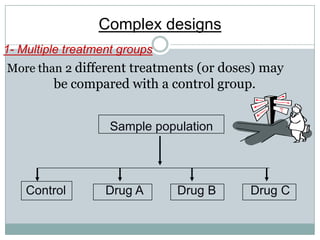
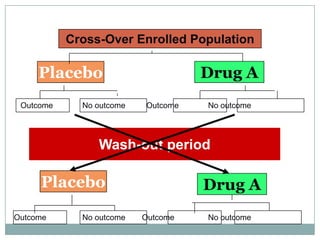
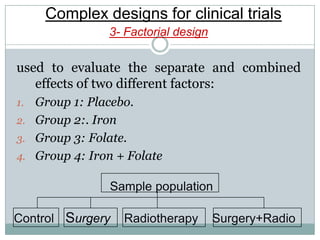
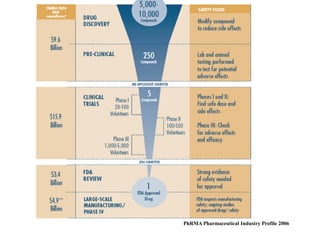
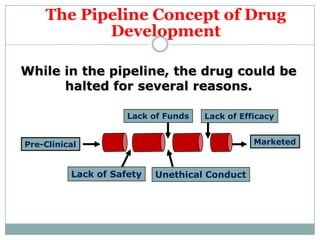
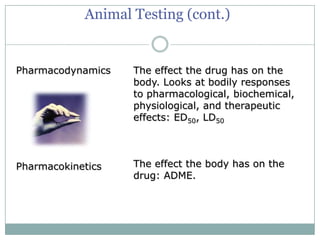
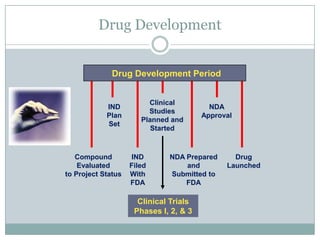
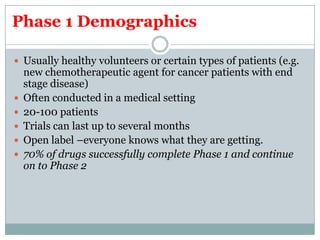
This document provides an overview of the methodology used in clinical trials. It defines key terms like randomized controlled trials, control groups, randomization, and blinding. It describes the various phases of clinical trials including phases 1-3 and post-marketing studies. Phase 1 trials test safety in healthy volunteers while phases 2 and 3 test efficacy in larger patient populations. The goals of each phase are explained as well as important demographic information. The document also outlines the drug development process from preclinical research through regulatory approval and commercialization.