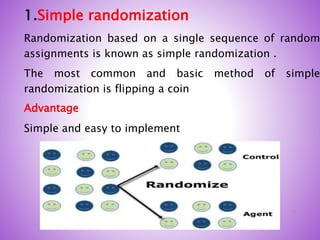
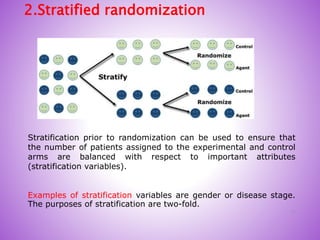
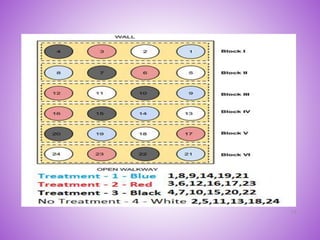
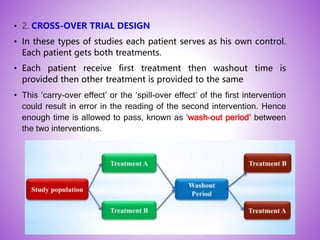
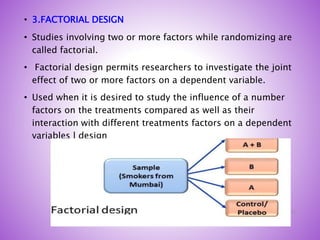
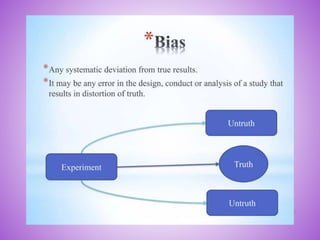
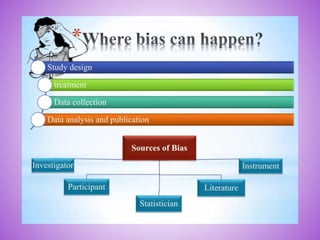
This document provides an overview of randomized controlled trials (RCTs). It discusses that RCTs are used to test interventions by randomly assigning participants to either an intervention or control group. The two groups are then compared on outcomes to see if any differences were caused by the intervention. It outlines the basic steps in RCTs, including developing a protocol, randomization methods, intervention/manipulation, follow-up, and outcome assessment. It also discusses types of RCT designs such as parallel group trials and crossover trials, as well as concepts like blinding and stratification.