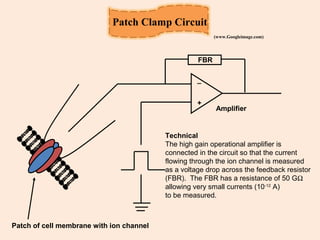
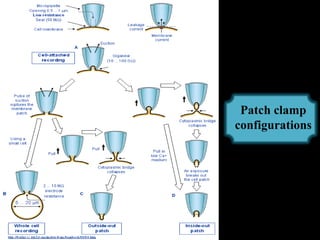
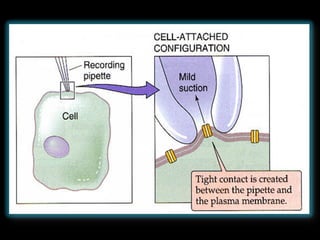
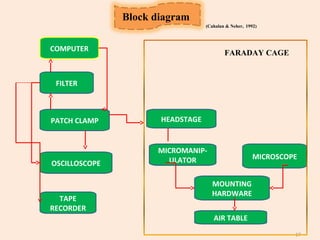
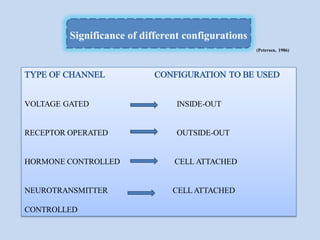
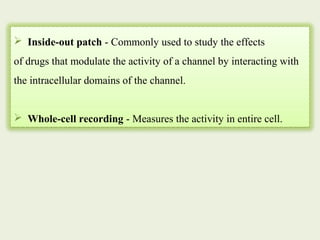
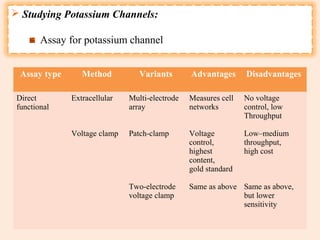
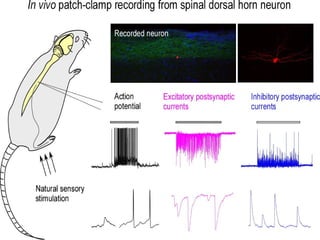
The document provides an overview of the patch clamp technique. It discusses the history, principle, method, configurations, instrumentation, and applications of patch clamp. Some key points include:
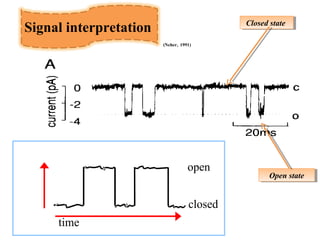
- Patch clamp allows the recording of currents through single ion channels in isolated patches of cell membrane.
- It was developed in 1976 by Erwin Neher and Bert Sakmann, who received the Nobel Prize for this work.
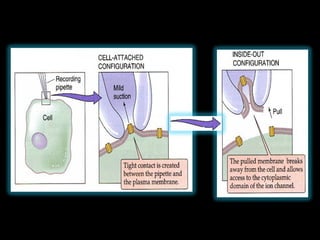
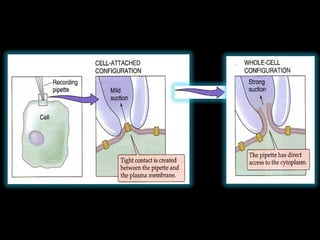
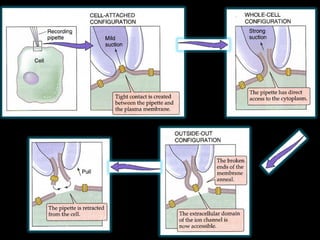
- Different configurations (whole-cell, outside-out, inside-out, cell-attached) are used depending on the question being studied.
- It is used to identify ion channels and study their properties like activation, permeability, and modulation by drugs.





![ 1963 It was concluded Ohm’s law can be applied to biological
system,
(V=Change in potential ,I=Current ,R=Resistance of system)
Itotal = IC + Iionic [ where IC= Capacitive current,
Iionic = Ionic current. ]
When dV/dt=0,
Itotal = Iionic [As IC = C.(dV/dt)]
V=IR
PRINCIPLE
(Hille, 1970 & Neher, 1991)](https://image.slidesharecdn.com/presentation1-160811083022/85/patch-clam-technique-6-320.jpg)