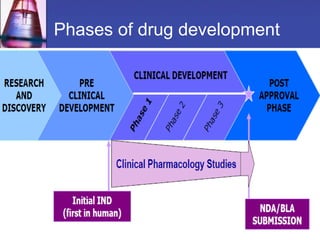
Phase 1 clinical trials are the first studies conducted in humans of a new drug or treatment. They aim to determine the drug's safety and tolerability, identify the maximum tolerated dose, and understand the drug's pharmacokinetics. Phase 1 trials typically involve small groups of healthy volunteers or patients and start with low doses that are gradually increased. The results of phase 1 trials provide information needed to design subsequent phase 2 and 3 trials to further evaluate efficacy.








![Method of First In Man study
Done in small number of healthy volunteers or in certain cases
patients
First in a small group of 20 to 25
Start with a dose adjusted from animal data
Slowly increase the dose to find a safe tolerated dose
If safe in a larger group of up to about 50 –75
Randomized , placebo controlled studies.
Performed by clinical pharmacologists
Performed after receiving ethics committee clearance and proper
regulatory approval.
Centre has emergency care & facility for kinetics study
Performed in a single centre
Takes 3 – 6 months [ 70% success rate]](https://image.slidesharecdn.com/phase1clinicaltrial-160804185720/85/Phase-1-clinical-trial-12-320.jpg)

![Execution of phase 1-Subject
selection
Inclusion criteria
Healthy volunteers ,
Uniformity of subjects
about age, sex,
nutritional status
[Informed consent a
must]
Exception:
Patients only for toxic
drugs Eg AntiHIV,
Anticancer
Exclusion criteria
Women of child bearing
age, children](https://image.slidesharecdn.com/phase1clinicaltrial-160804185720/85/Phase-1-clinical-trial-16-320.jpg)