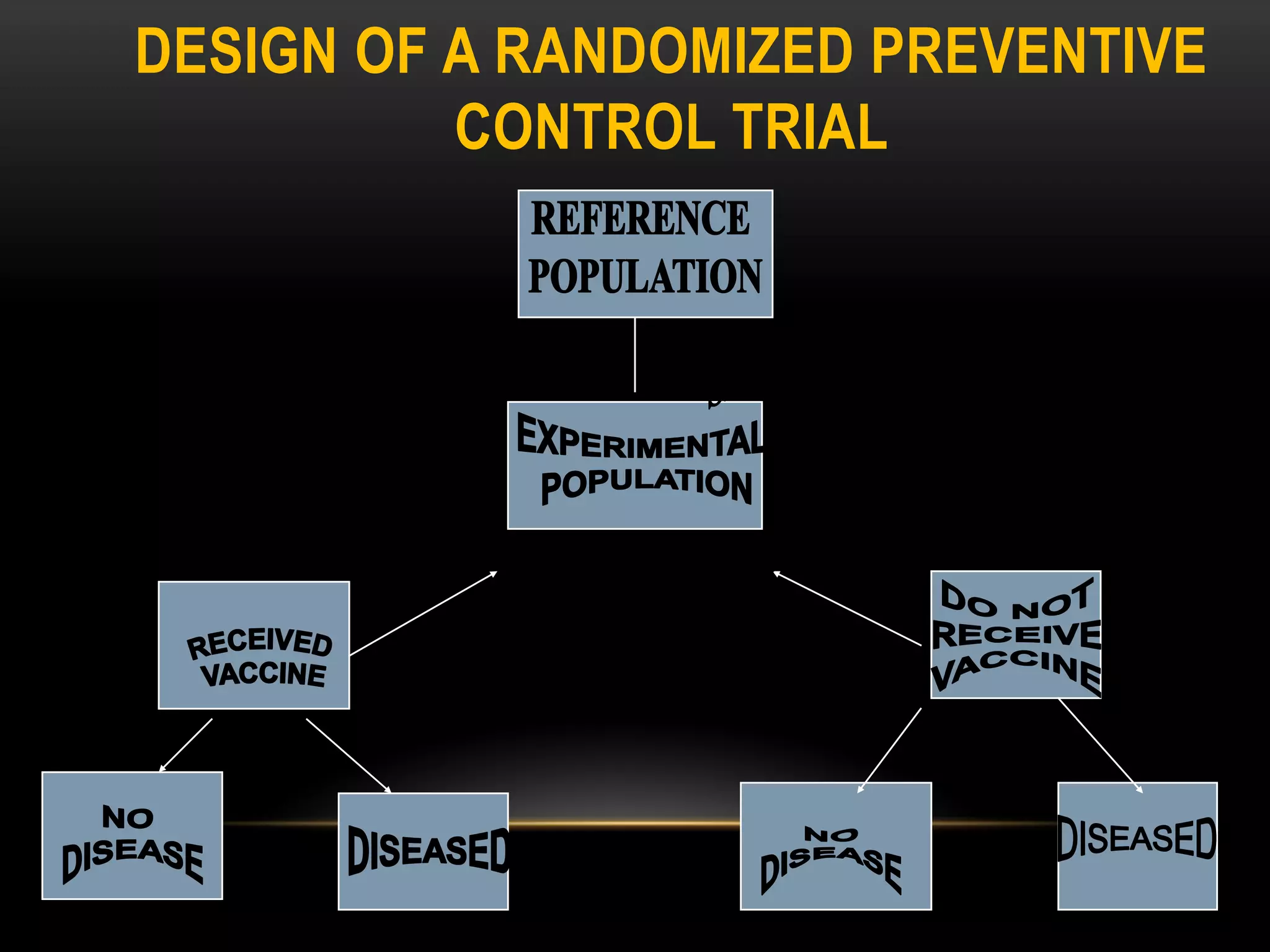
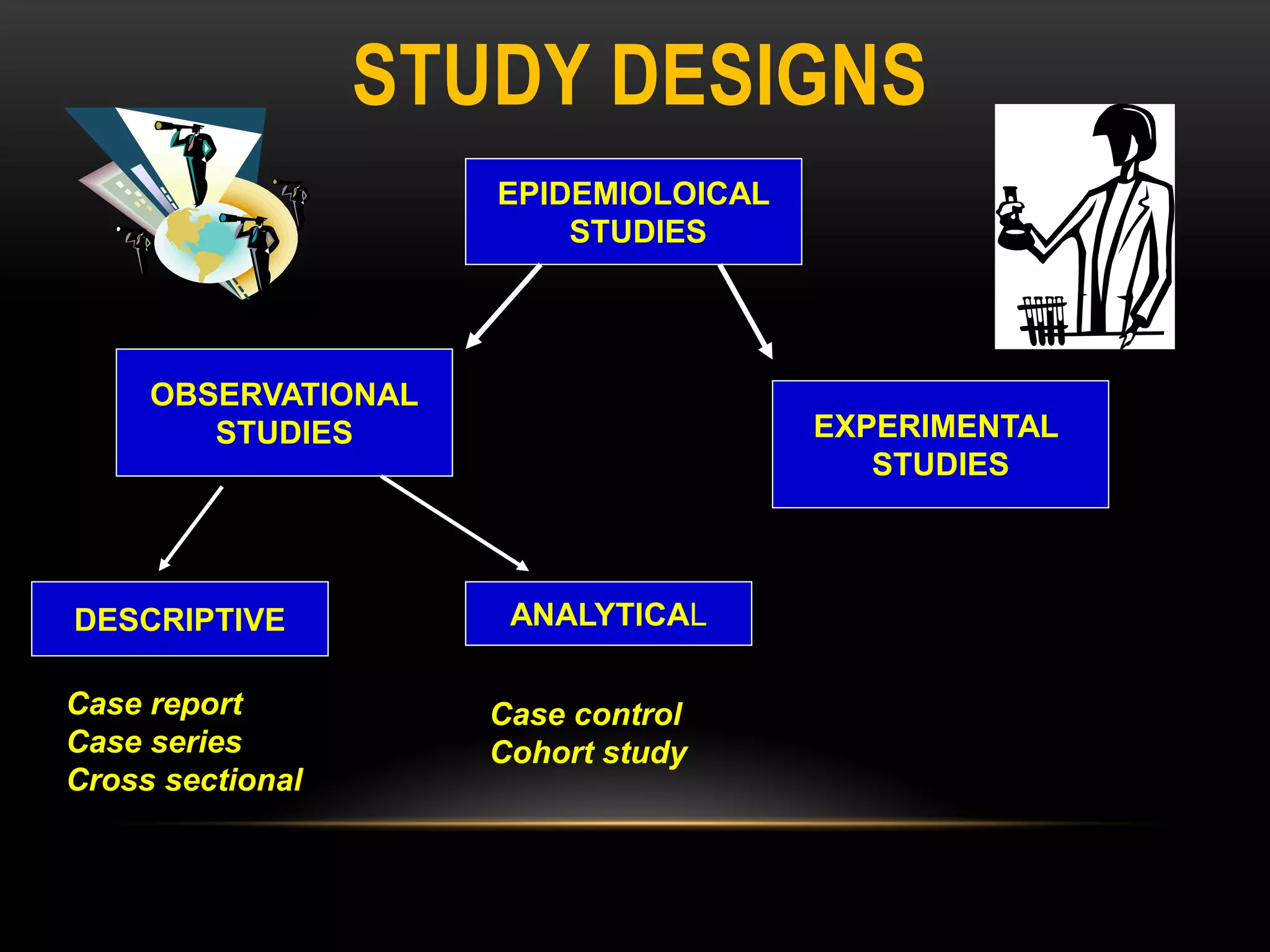
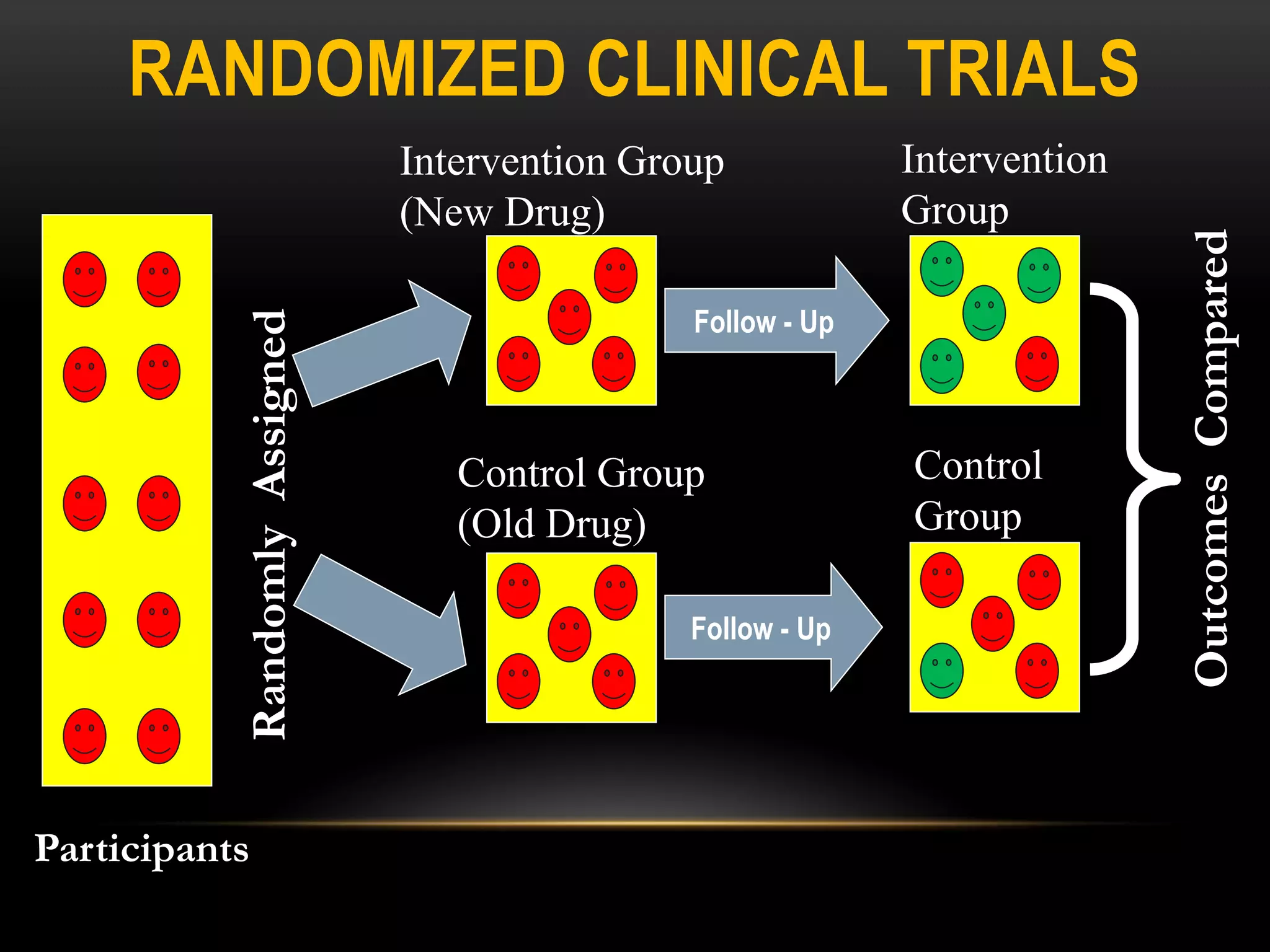
The document discusses experimental study designs and randomized clinical trials. It provides information on the different phases of clinical trials including objectives, demographics, and goals. Phase I trials test safety and dosing in small groups. Phase II explores biological effects and estimates response rates. Phase III confirms efficacy in larger and more diverse populations to provide evidence for marketing approval. Randomization, blinding, and placebos are used to reduce bias. Experimental studies directly manipulate variables under controlled conditions to measure outcomes compared to control groups.












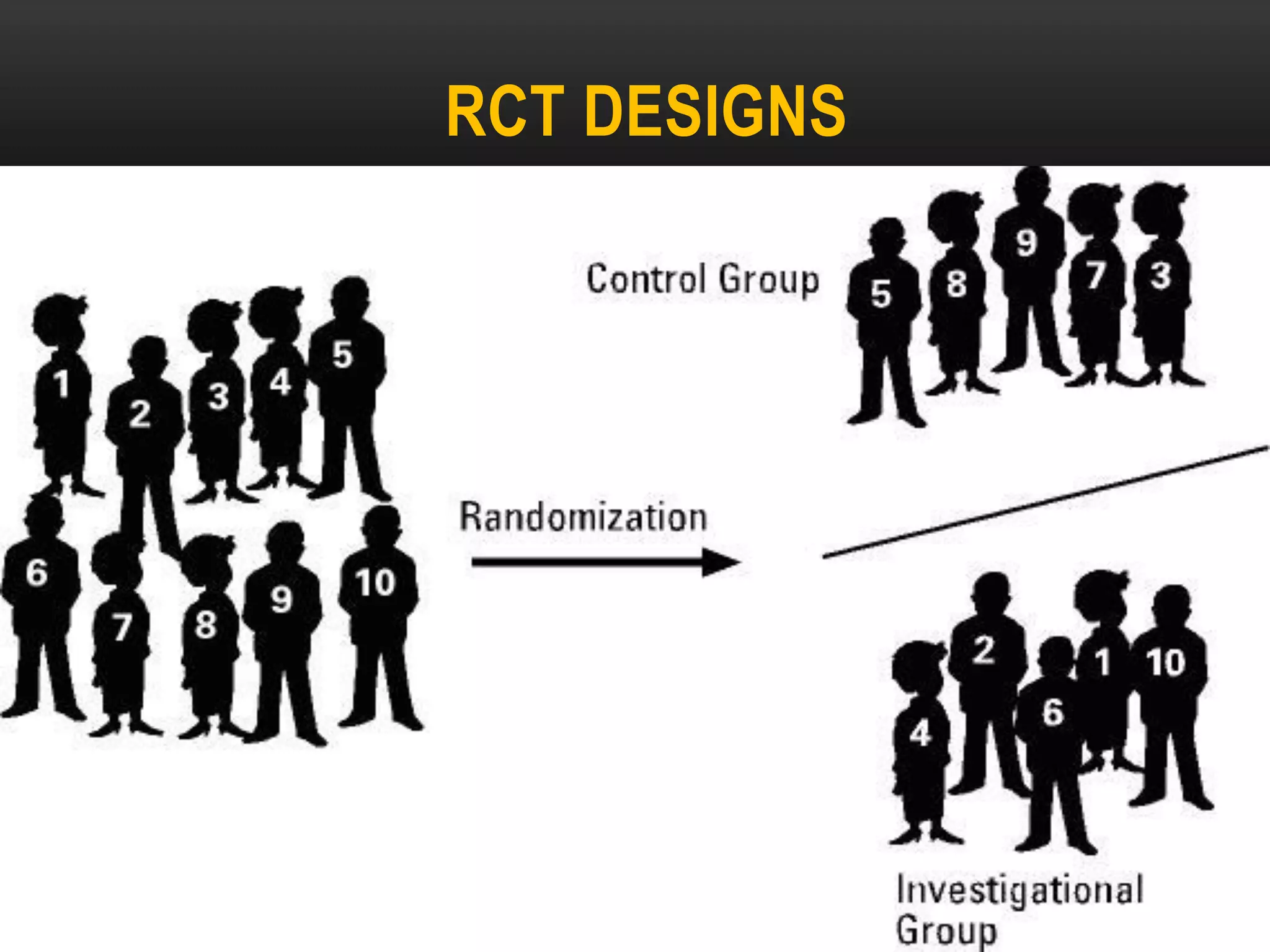
![RCT DESIGNS
[1] According to exposure to intervention:
Parallel design Each group is subjected one
intervention ( the most common design)
Group A
Group B
TREATMENT A
TREATMENT B / Placebo](https://image.slidesharecdn.com/lecture10experimentalstudy-1-221021221616-bec7e291/75/Lecture-10-Experimental-study-1-pdf-17-2048.jpg)
![RCT DESIGNS
[1] According to exposure to intervention:
Cross – Over design Each participant is given
both interventions in successive periods. Each
participant acts as his or her own control.
Treatment Placebo
TREATMENT A TREATMENT B](https://image.slidesharecdn.com/lecture10experimentalstudy-1-221021221616-bec7e291/75/Lecture-10-Experimental-study-1-pdf-18-2048.jpg)
![RCT DESIGNS
[1] According to exposure to intervention
Factorial design: When interventions are
compared separately, in combination and
against a control in different groups.
TREATMENT A
TREATMENT B
TREATMENT A +B
PLACEBO
Group A
Group B
Group C
Group D](https://image.slidesharecdn.com/lecture10experimentalstudy-1-221021221616-bec7e291/75/Lecture-10-Experimental-study-1-pdf-19-2048.jpg)

![RCT DESIGNS
[2] According to “Blindness” to the interventions
Open RCT everybody involved in the trial knows which
intervention is given to each participant
Single blind RCT either the participants or the investigators do
not know the identity of interventions
Double blind RCT Both the participants or the investigators do
not know the identity of the interventions
Triple blinded RCT: Participant, investigators or person who
evaluates do not know the identity of the intervention](https://image.slidesharecdn.com/lecture10experimentalstudy-1-221021221616-bec7e291/75/Lecture-10-Experimental-study-1-pdf-21-2048.jpg)





































![PHASE IV OR POST MARKETING
SURVEILLANCE
• No fixed duration / patient population
• Starts immediately after marketing
• Report all acute adverse reactions (ADRs)
• Helps to detect
• Rare ADRs
• Drug interactions
• Also new uses for drugs [Sometimes called Phase V]
• Do not involve control groups to evaluate properly the
proper role of an intervention](https://image.slidesharecdn.com/lecture10experimentalstudy-1-221021221616-bec7e291/75/Lecture-10-Experimental-study-1-pdf-60-2048.jpg)