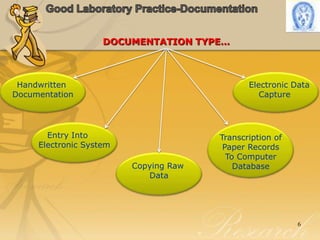
This document discusses the importance of documentation in Good Laboratory Practice (GLP). It emphasizes that documentation provides an audit trail and shows what actually occurred during a study, which is critical for validating study results. Poor or missing documentation can invalidate a study and lead to regulatory issues. The key requirements for GLP documentation are that it must be identifiable, prompt, accurate, legible, and signed/dated. Corrections to documentation must also be explained, signed, and dated. Proper documentation practice is paramount to demonstrating compliance with GLP standards.