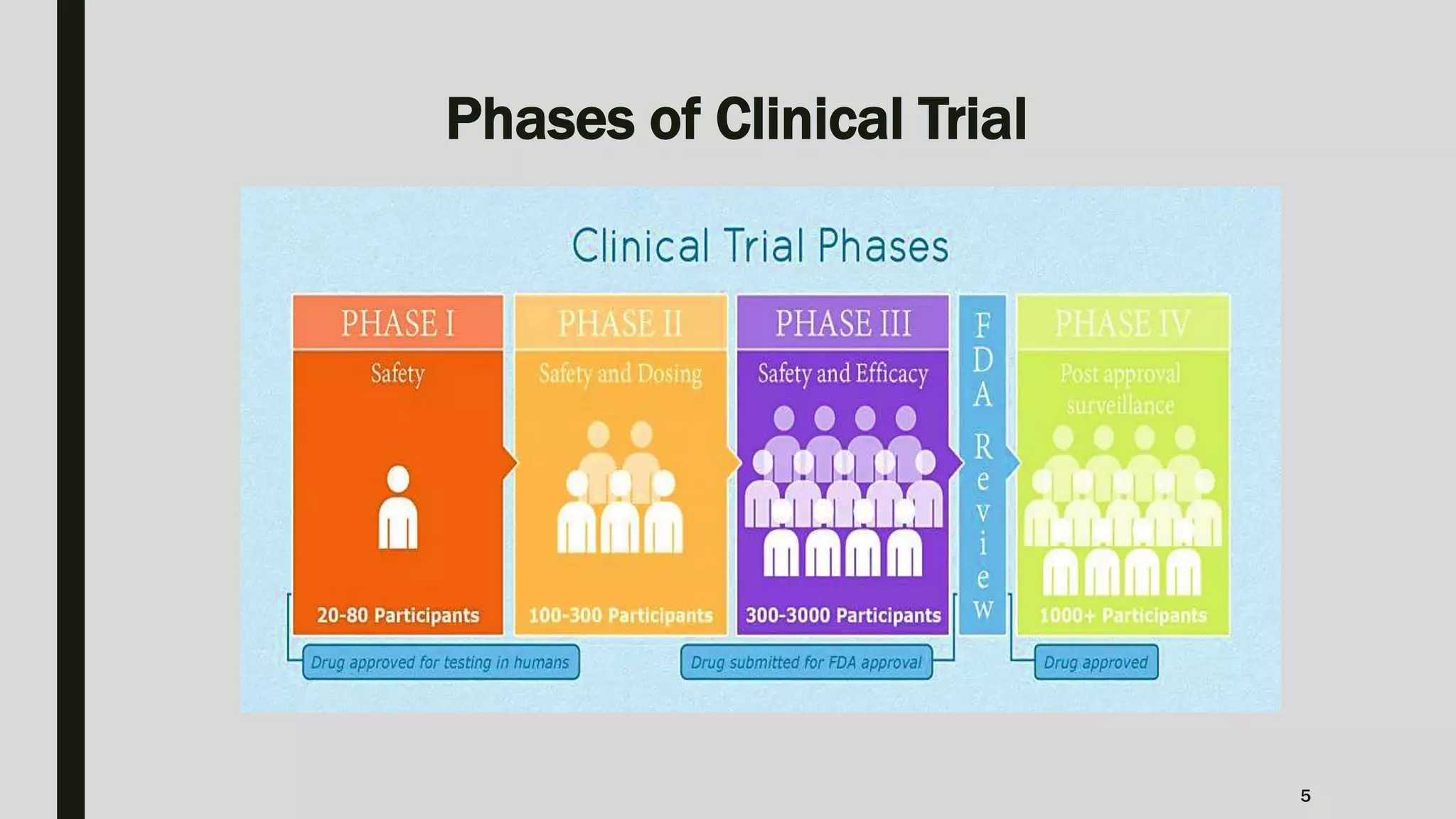
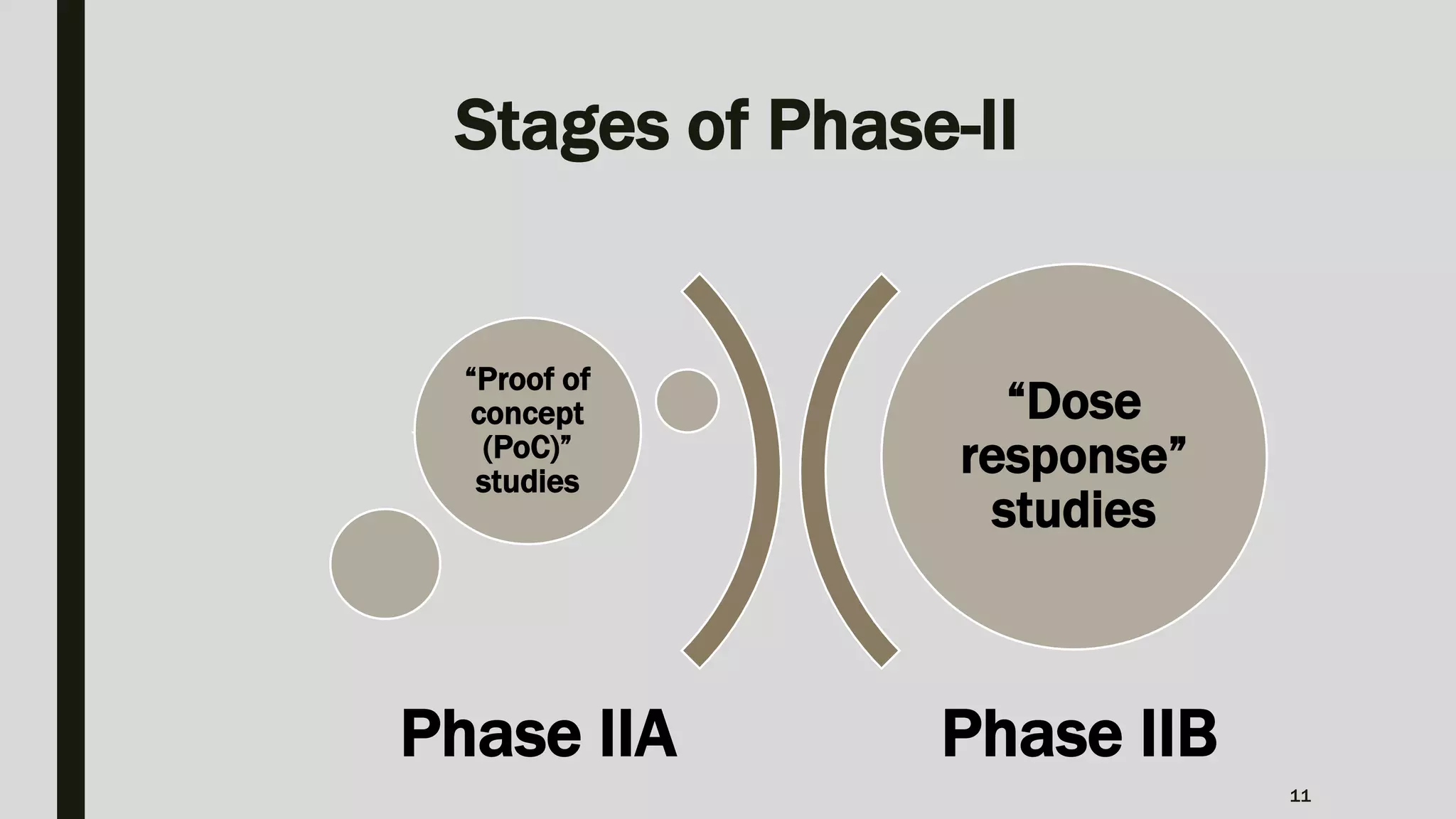
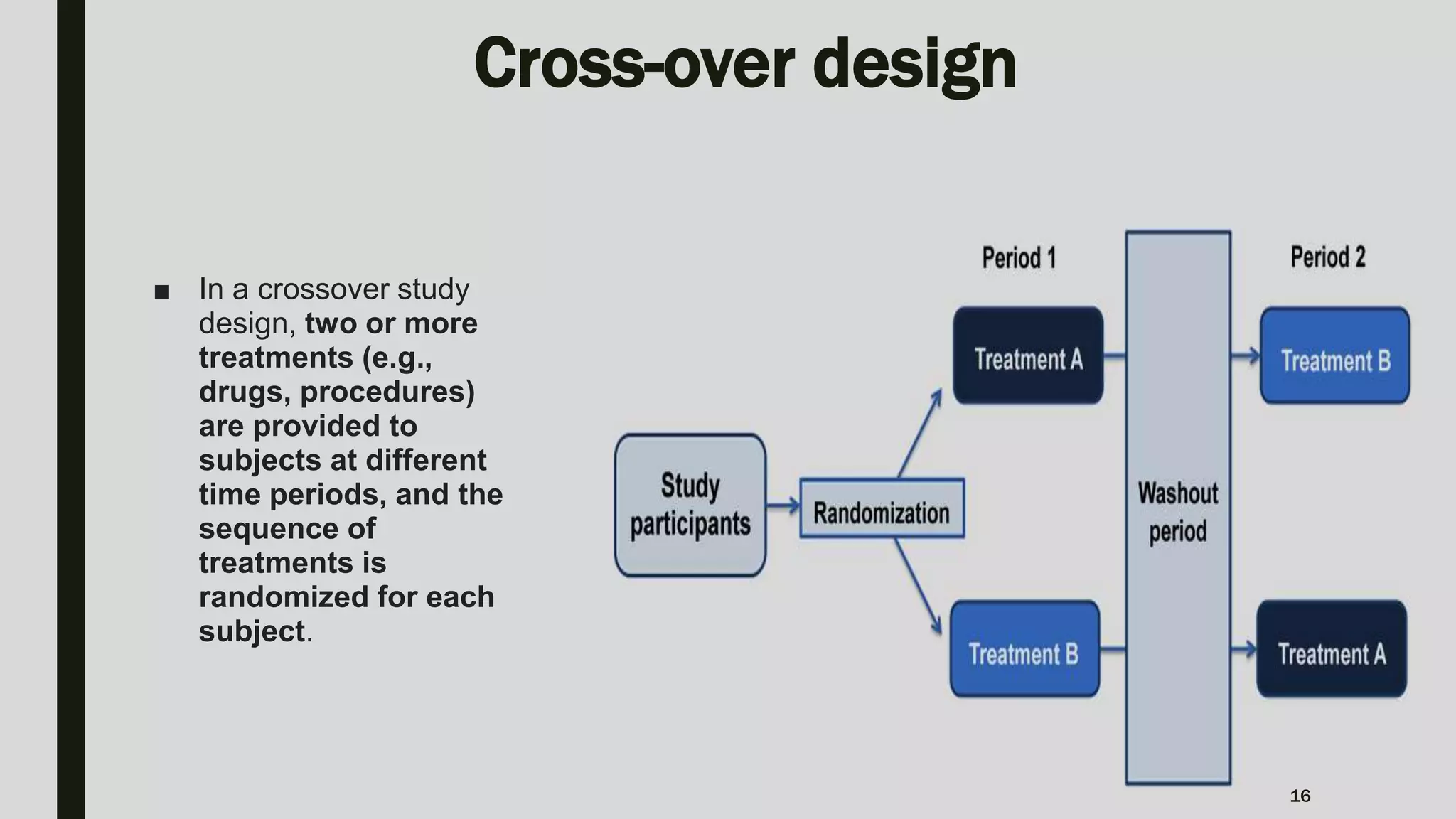
The document summarizes key aspects of Phase II clinical trials. It discusses that Phase II trials involve 100-400 patients and aim to establish therapeutic efficacy, determine optimal dosing, and further evaluate safety and tolerability. Randomized, cross-over, and platform trial designs may be used. Eligible patients typically have the target disease or condition. Sample size calculations are performed to ensure sufficient statistical power. Phase II trials generally last several months to 2 years.