The document outlines various practices related to Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP) aimed at ensuring quality and compliance in scientific research and manufacturing. It details principles, historical regulations, and quality assurance measures necessary for reliable scientific outcomes and safe products. Additionally, it addresses potential misconduct and emphasizes the importance of documentation and training in maintaining ethical standards in these practices.















![Access 2 Perspectives 2017
W. Edwards Deming
(1900-1993)1. Better design of products to improve service
2. Higher level of uniform product quality
3. Improvement of product testing in the workplace and in research centers
4. Greater sales through side [global] markets
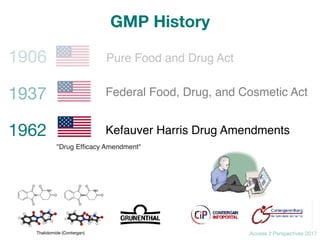
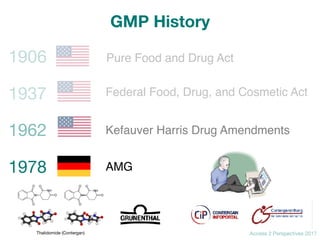
GMP History](https://image.slidesharecdn.com/gmp2daycourse2017access2perspectives-171027110203/85/Good-Manufacturing-Practice-GMP-2day-course-19-320.jpg)