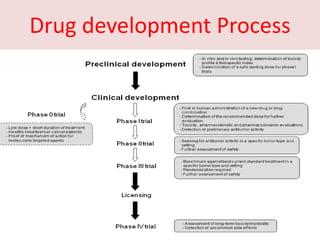
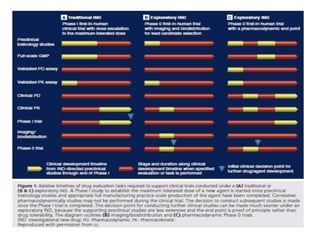
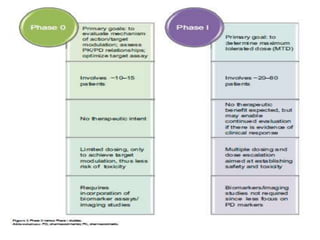
This document discusses phase 0 clinical trials, also known as microdosing studies. Phase 0 trials involve administering very low, non-therapeutic doses of an experimental drug to small groups of subjects to obtain preliminary pharmacokinetic and pharmacodynamic data before proceeding to full phase 1 trials. The goals are to accelerate drug development, eliminate unsuitable drug candidates early to save costs and reduce unnecessary risk to subjects and use of animals in testing. Regulatory guidelines have been established for microdosing studies. While microdosing offers benefits, limitations include potential for false predictions and difficulty motivating subject participation due to lack of intended therapeutic benefit. Ethical considerations also need to be addressed for these early-stage trials.