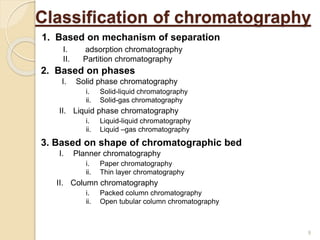
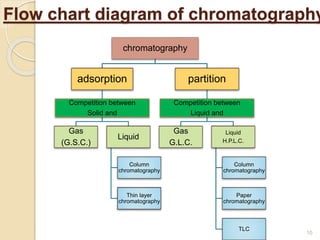
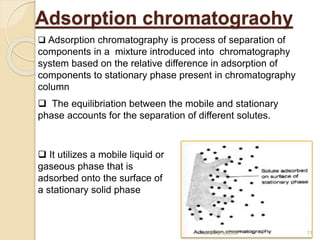
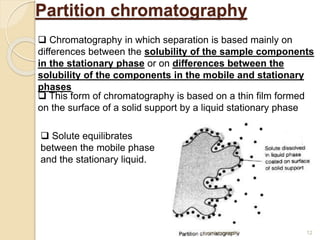
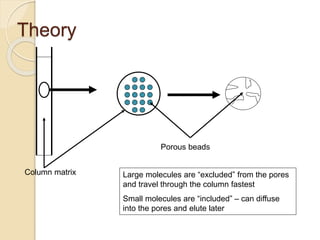
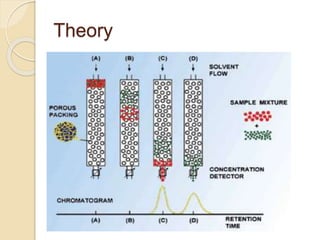
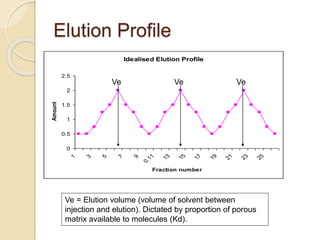
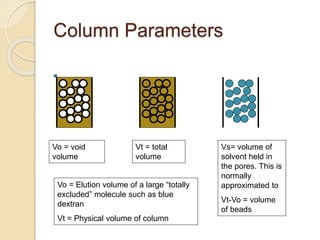
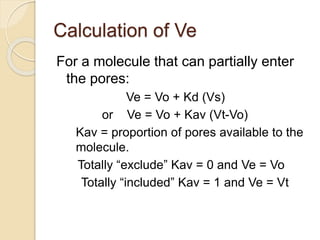
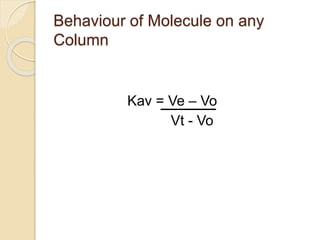
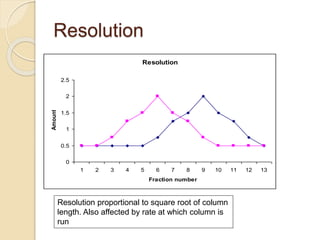
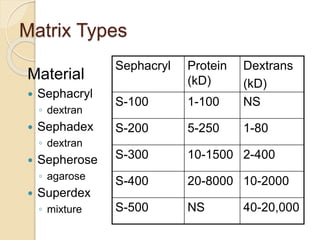
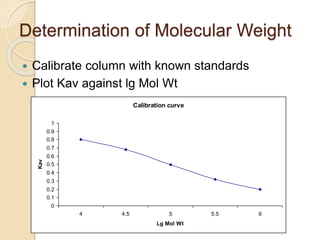
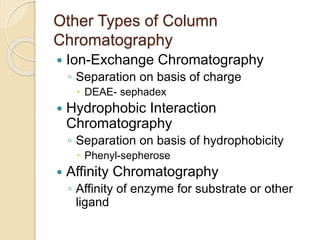
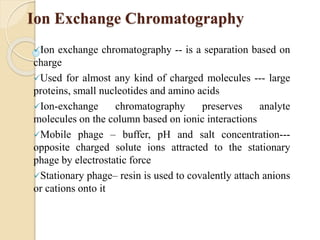
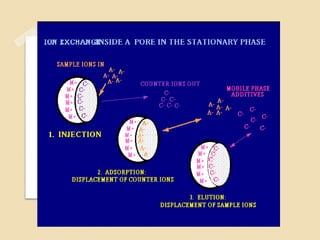
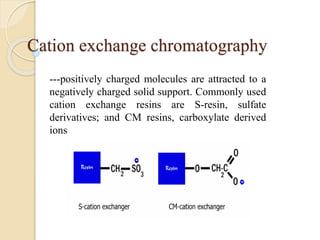
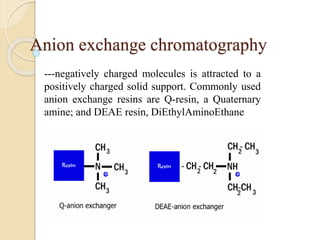
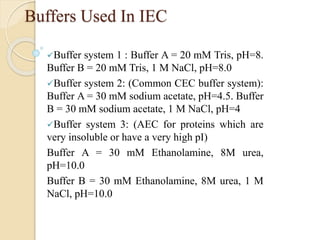
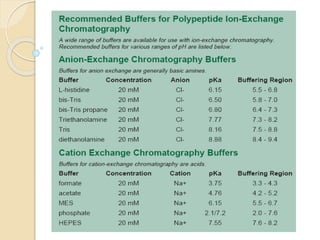
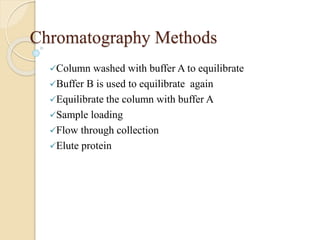
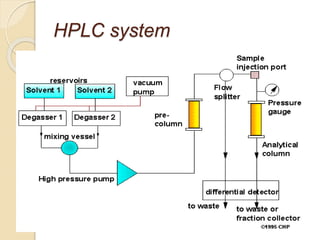
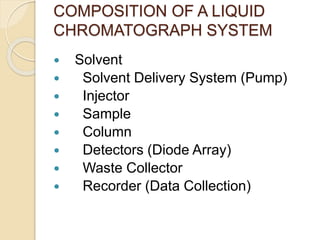
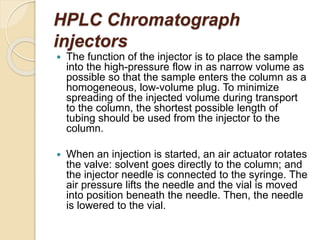
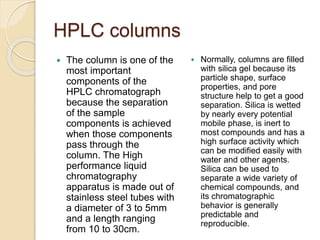
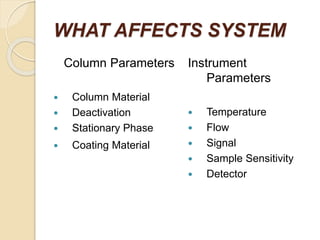
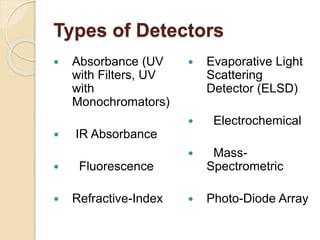
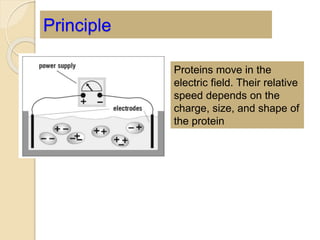
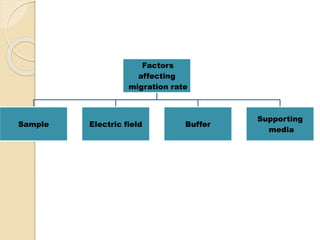
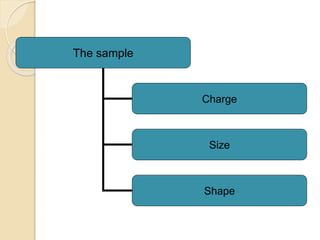
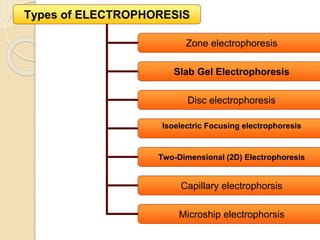
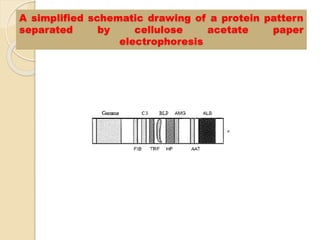
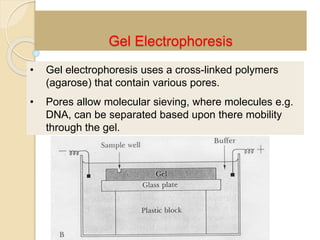
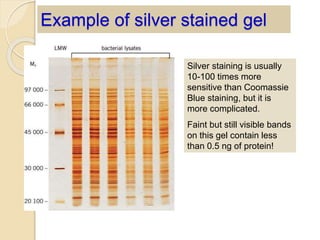
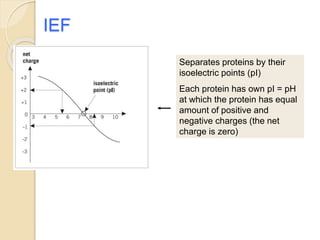
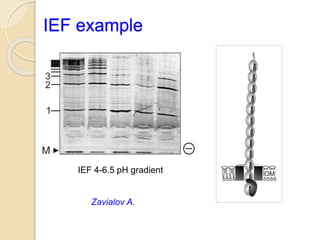
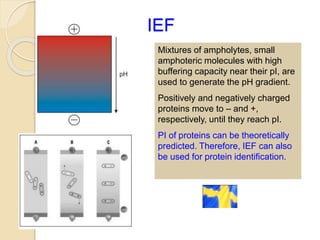
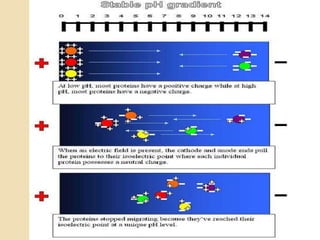
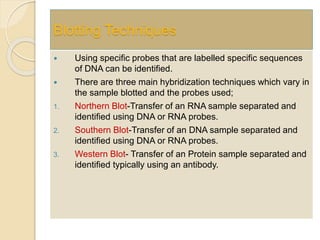
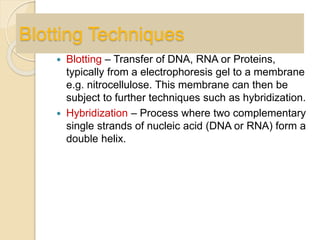
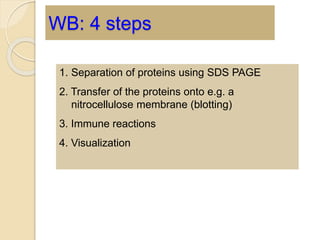
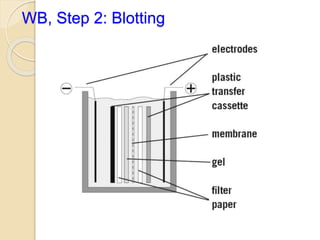
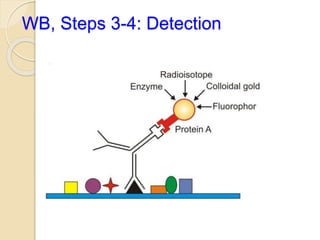
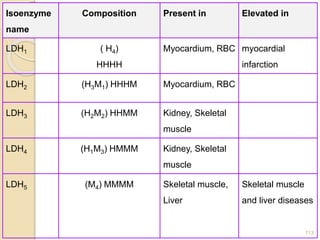
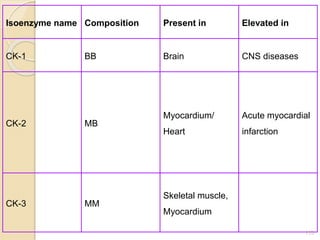
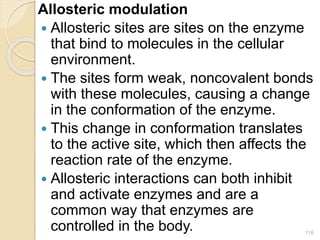
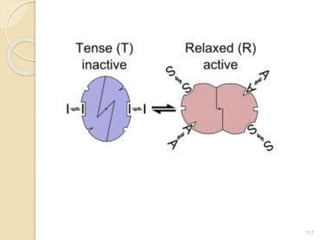
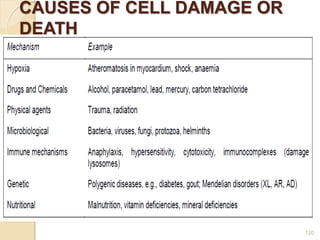
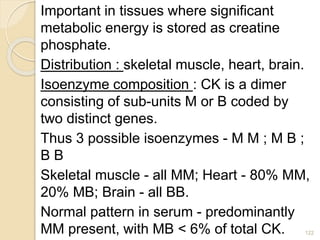
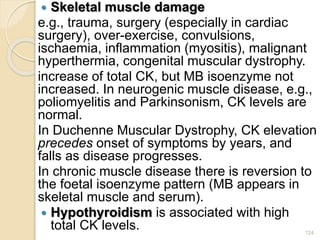
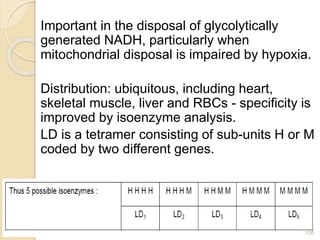
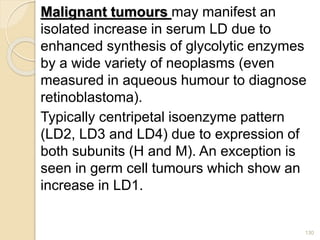
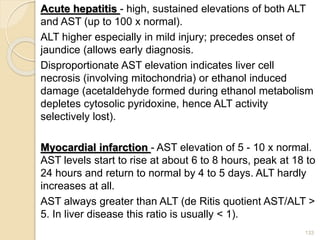
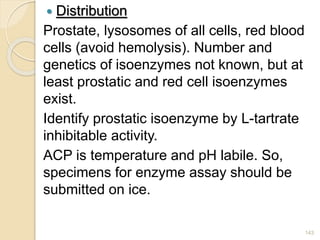
The document provides an extensive overview of chromatography, including its introduction, history, principles, applications, and various separation techniques such as adsorption, partition, gas-liquid, and liquid-liquid chromatography. It details the components and processes involved in chromatography techniques and discusses the importance of different phases, properties of stationary phases, and chromatographic terms. Additionally, the document covers specific methods like ion-exchange chromatography and high-performance liquid chromatography (HPLC), highlighting their uses in research, quality control, and environmental monitoring.