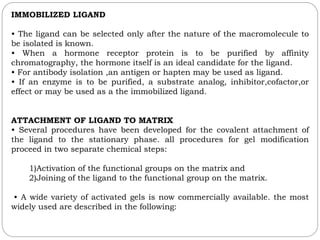
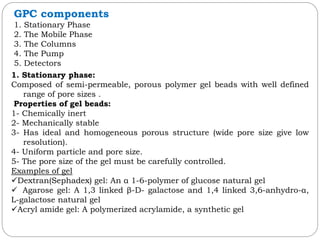
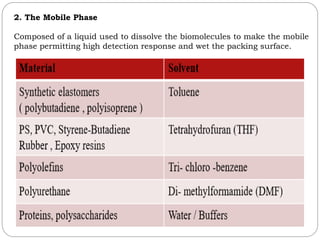
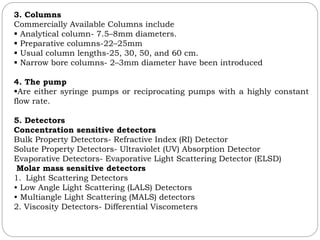
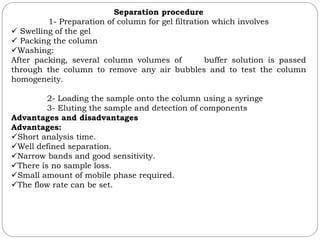
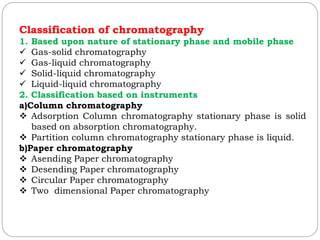
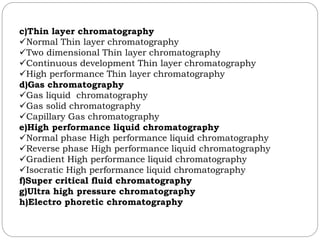
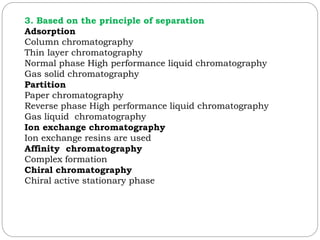
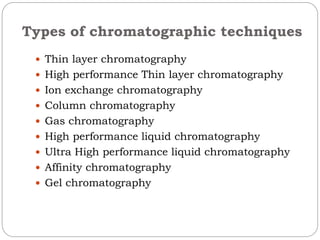
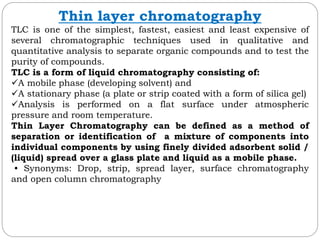
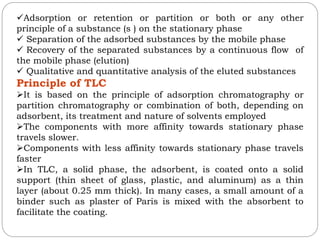
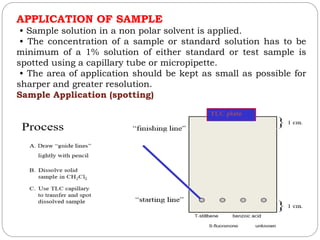
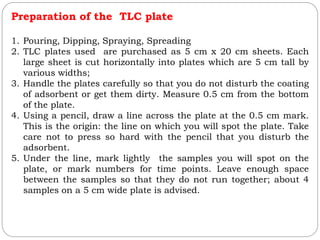
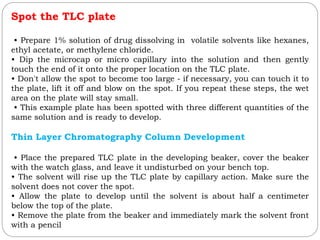
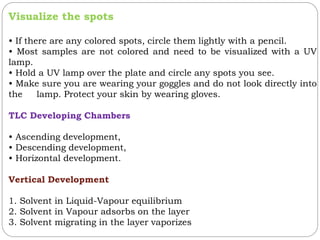
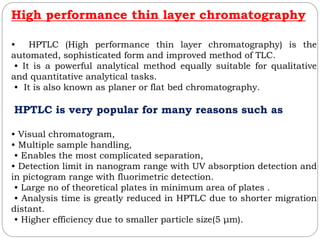
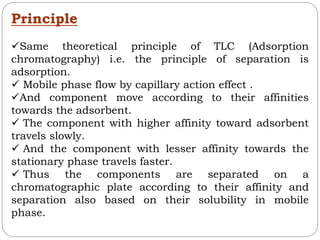
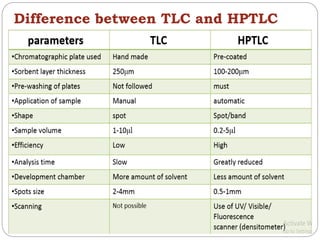
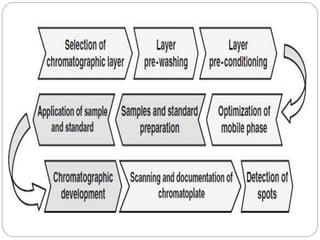
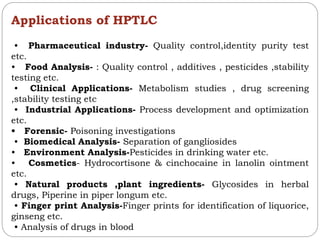
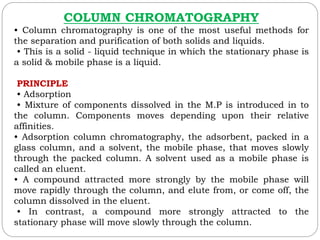
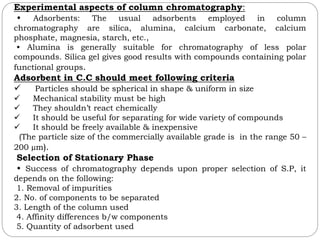
This document provides an overview of chromatography techniques. It defines chromatography as a physical separation method that distributes components between two phases, one stationary and one mobile. It then classifies chromatography based on the stationary and mobile phases used as well as the instruments involved. Several chromatography techniques are described in detail, including thin layer chromatography. The principles, components, preparation, and considerations for thin layer chromatography are explained.
![Presented by : k. jayalakshmi
D/o k. kristaiah
1st yr M.pharm, pharmaceutical analysis
Raghavendhra institute of pharmaceutical education and research
[RIPER], AUTONOMOUS
Chromatography](https://image.slidesharecdn.com/chromatography-191207175351/75/Chromatography-1-2048.jpg)















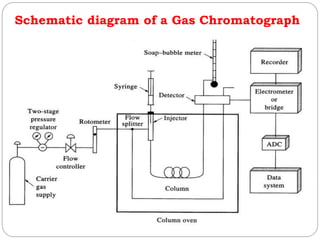
![Carrier gas
The carrier gas must be chemically inert.
Commonly used gases include nitrogen, helium, argon, and carbon dioxide.
The choice of carrier gas is often dependant upon the type of detector which
is used.
The carrier gas system also contains a molecular sieve to remove water and
other impurities.
P inlet 10-50 psig
F=25-150 mL/min packed column
F=1-25 mL/min open tubular column
Sample injection- Direct Injection
Direct injection :into heated port (>T oven) using micro syringe
(i) 1-20 uL packed column
(ii) 10-3 uL capillary column
Sample injection- rotary sample valve with sample loop
Split injection: routine method
0.1-1 % sample to column
remainder to waste
Split less injection: all sample to column
best for quantitative analysis
only for trace analysis, low [sample]](https://image.slidesharecdn.com/chromatography-191207175351/85/Chromatography-62-320.jpg)

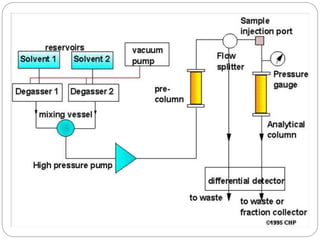
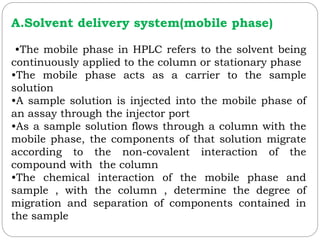
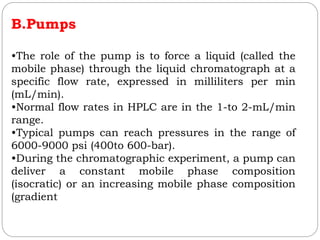
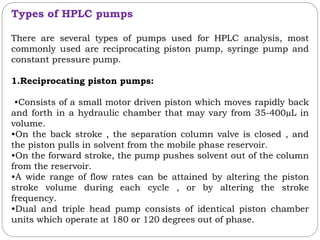

![2.Detector and Injector linearity
Column : ODS or C18
Mobile phase : milli Q water and acetonitrile (80:20)
Flow rate: 1ml/min
Temperature: 40 Centigrade
Detector wavelength:272 nm
Runtime:10min
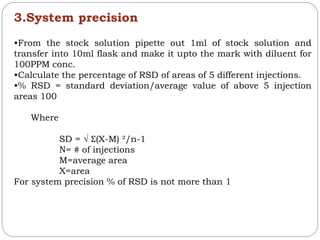
Preparation of stock solution:
•Take 50mg of caffeine and transfer into 50ml of flask and make it
up to the mark with the diluents.
•Caffeine is used a it gives a multiwavelength response and is stable
•Prepare solutions of different PPM (100-600)
•Inject them and calculate the area
•Correlation coefficient r is used to check the detector linearity and
cannot be less than .999
• r = NƩXY-ƩXƩY/√ [NƩX²-(Ʃx) ²][NƩY²-(ƩY)²]
•The graph obtained between concentration and area is linear.](https://image.slidesharecdn.com/chromatography-191207175351/85/Chromatography-89-320.jpg)