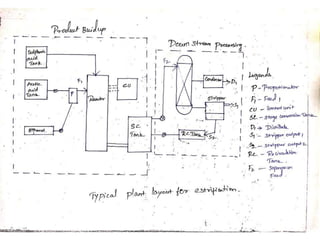
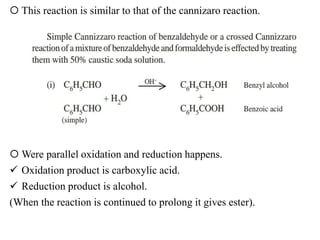
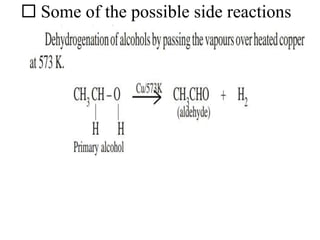
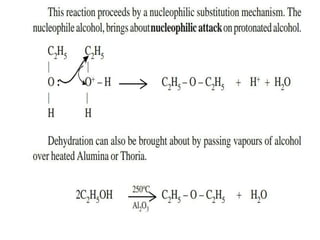
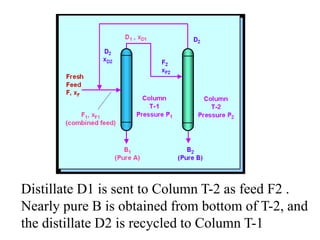
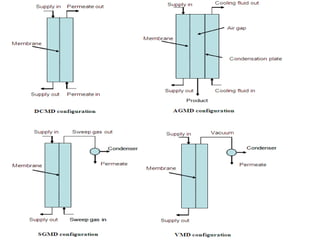
Ethyl acetate is an organic compound (C4H8O2) used primarily as a solvent in various industries, including the decaffeination of tea and coffee. It is a colorless liquid with a fruity odor, has low toxicity, and can cause irritation upon overexposure. Production methods include Fischer esterification, Tishchenko reaction, and dehydrogenation of ethanol, each with unique advantages and byproducts.