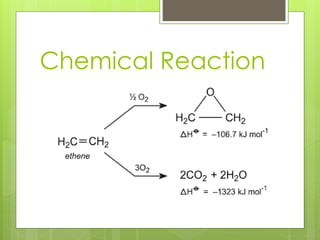
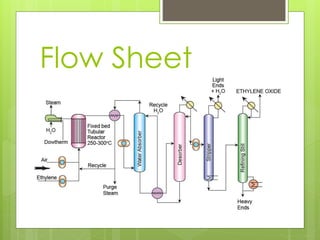
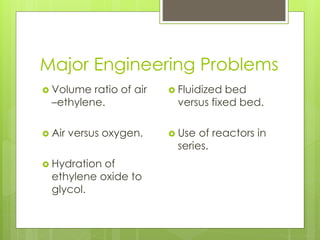
Ethylene oxide is produced through the direct oxidation of ethylene using a silver oxide catalyst between 250-300°C and 4-5 atmospheres of pressure. Ethylene and air are mixed and passed over the catalyst, and the highly exothermic reaction produces ethylene oxide with a 60-70% yield. The effluent gases are washed and the absorbed ethylene oxide is separated through fractional distillation, producing a 95-98% pure product. Major engineering challenges include controlling the air-to-ethylene ratio and preventing the competitive oxidation reaction to carbon dioxide and water.