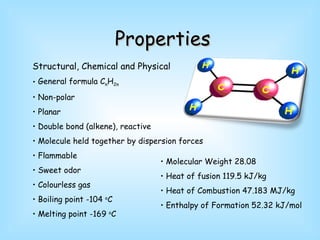
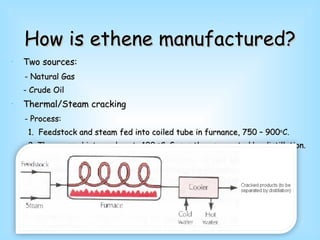
Ethene is a colorless gas with the formula C2H4 that is mainly produced through the cracking of petroleum products and natural gas. It undergoes characteristic alkene reactions like addition and oxidation. Ethene is an important industrial chemical used to produce polymers like polyethene, polyvinyl chloride, and polystyrene that are used to make various plastic products, as well as in other applications like fruit ripening and as an antifreeze component.