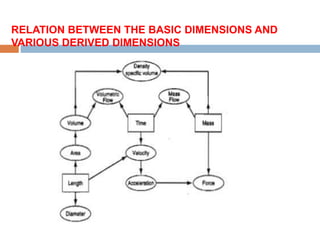
The document discusses the principles of units and dimensions in chemical engineering, explaining the difference between fundamental and derived units. It describes various systems of units, including SI, CGS, FPS, and BE, alongside their practical benefits and operational rules for unit conversions. Additionally, the document provides examples and exercises related to the conversion of units and their application in problem-solving.







![ Dyne: One dyne is the force necessary to impart to a mass of an
acceleration of 1cm/s2.
F =ma
Dyne = 1gm.cm/s2 = gm.cm s-2
= [MLT-2]
Newton: Newton is the force necessary to impart to a mass of 1 Kg
an acceleration of 1m/s2.
F = ma
N = m/s2 = Kgms-2 = [MLT-2]
Joule: One joule is amount of work done when a force of 1N acting
on a body displaces it through a distance of 1m along the direction
of force i.e.
Joule = N .m = Kg m/s2 .m = Kgm2. S-2
1 joule = 107 erg J > erg
Erg: It is the amount of work done when a force of I dyne acting on
body displaces it through a distance of 1cm along the direction of
force.](https://image.slidesharecdn.com/chemicalprincipleprocess-170419123733/85/Chemical-principle-process-10-320.jpg)