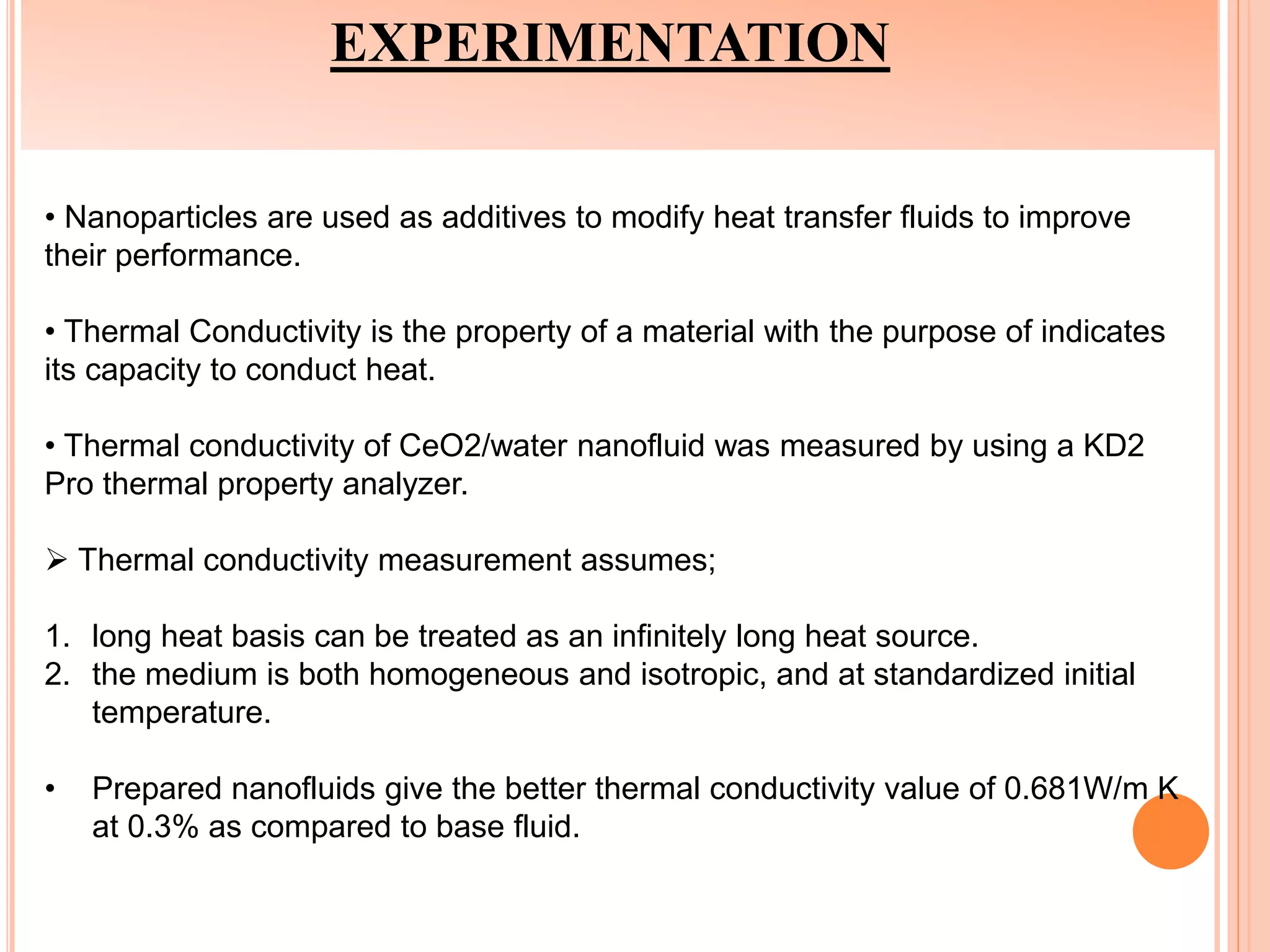
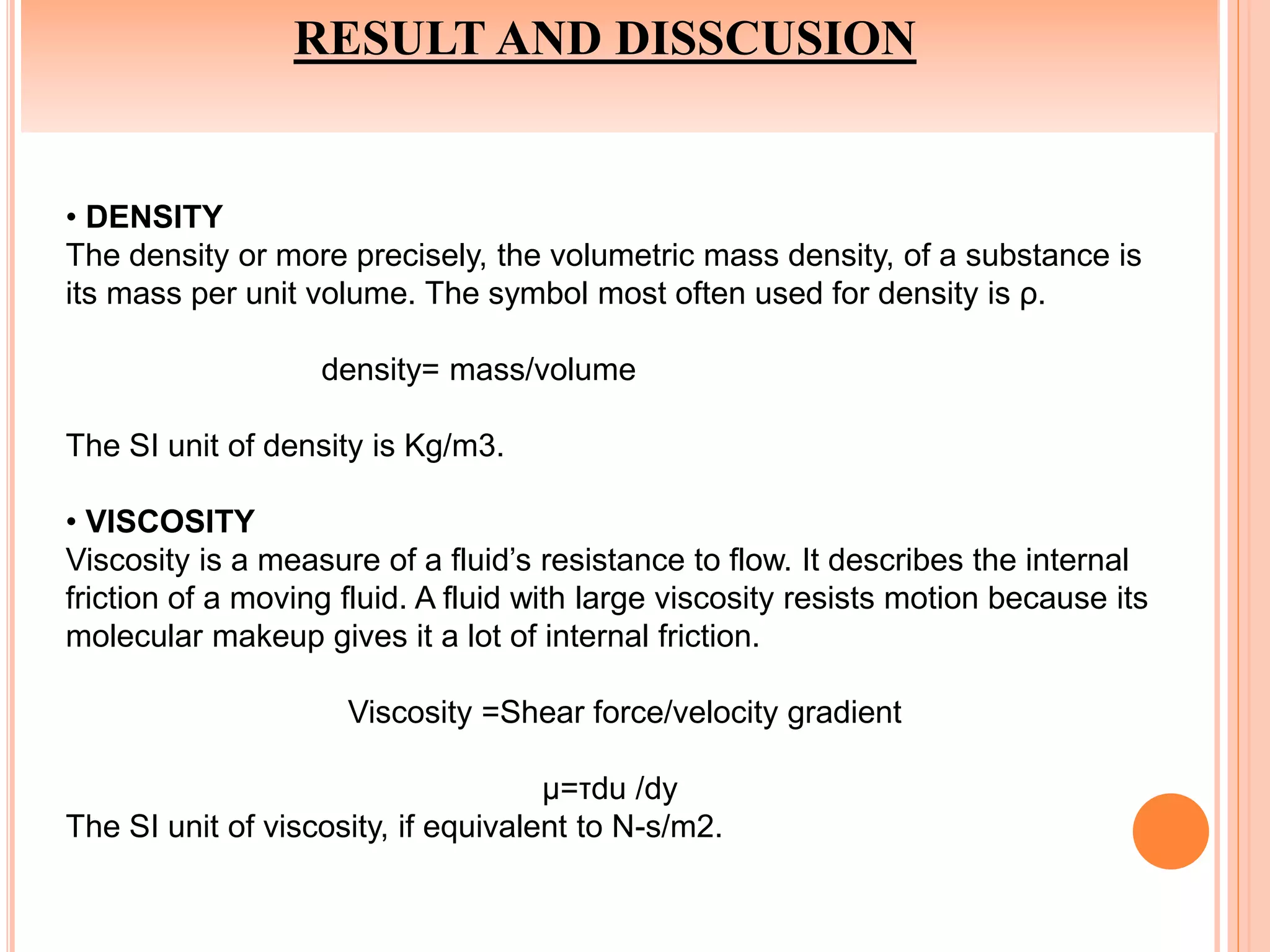
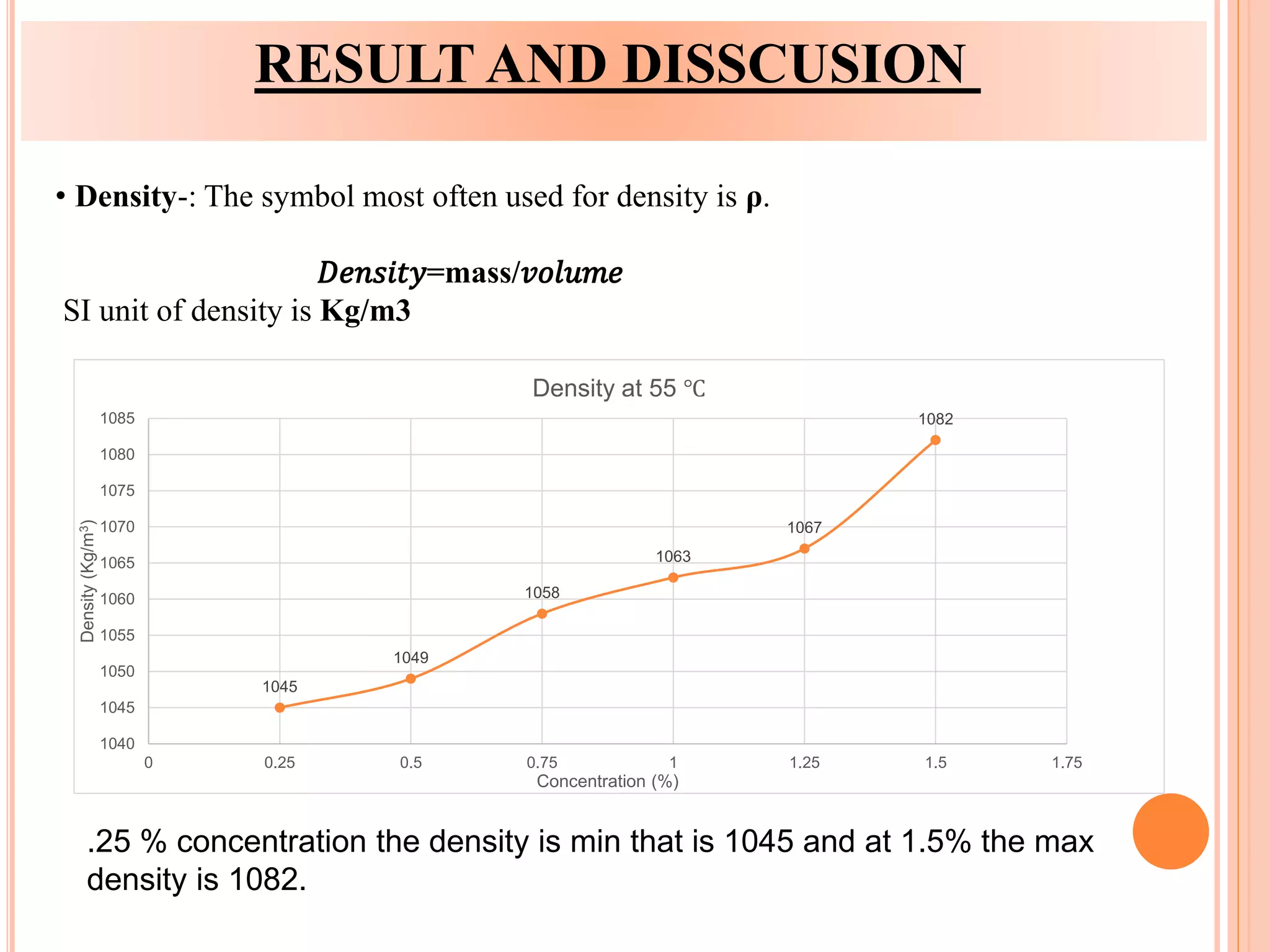
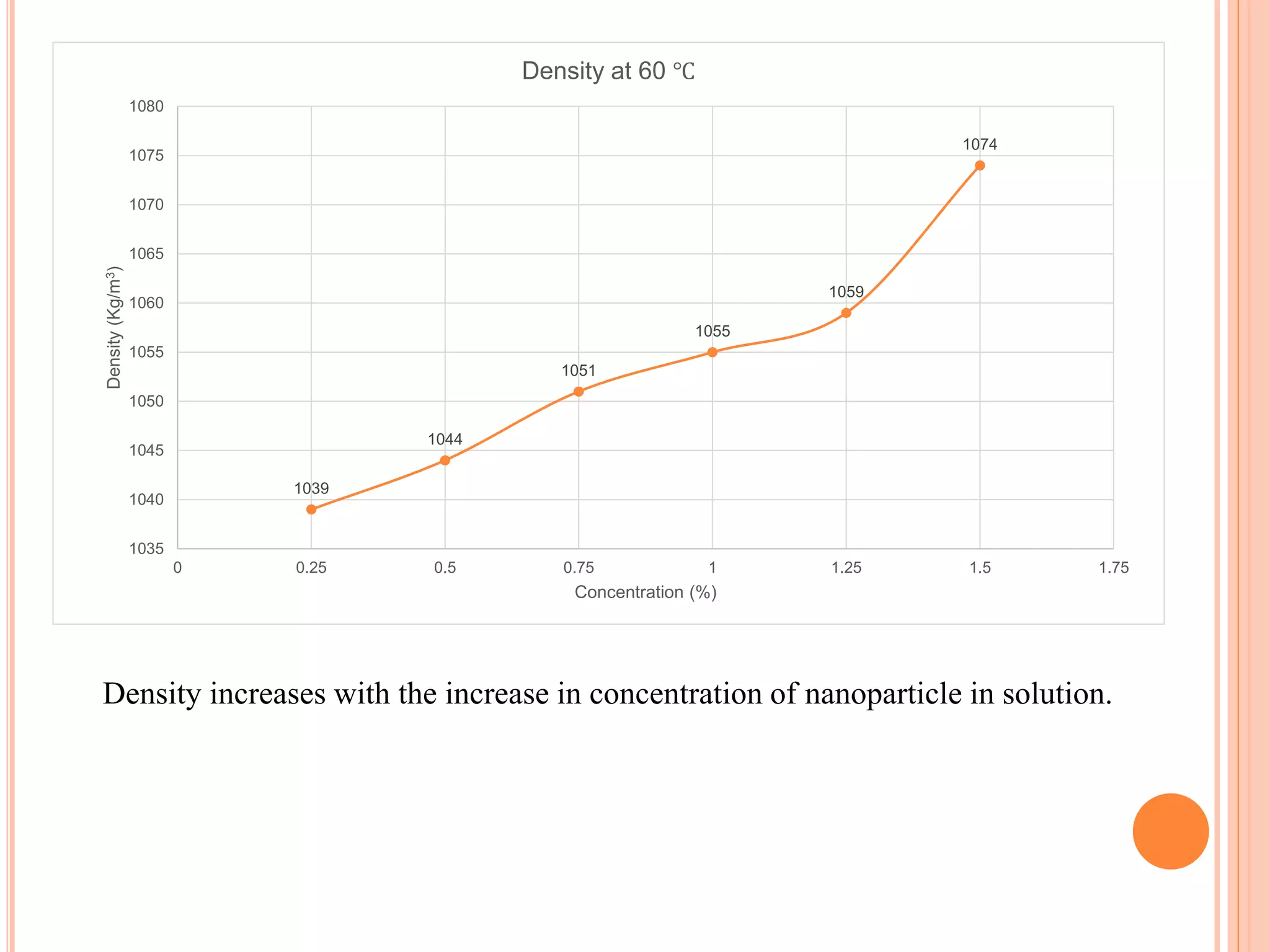
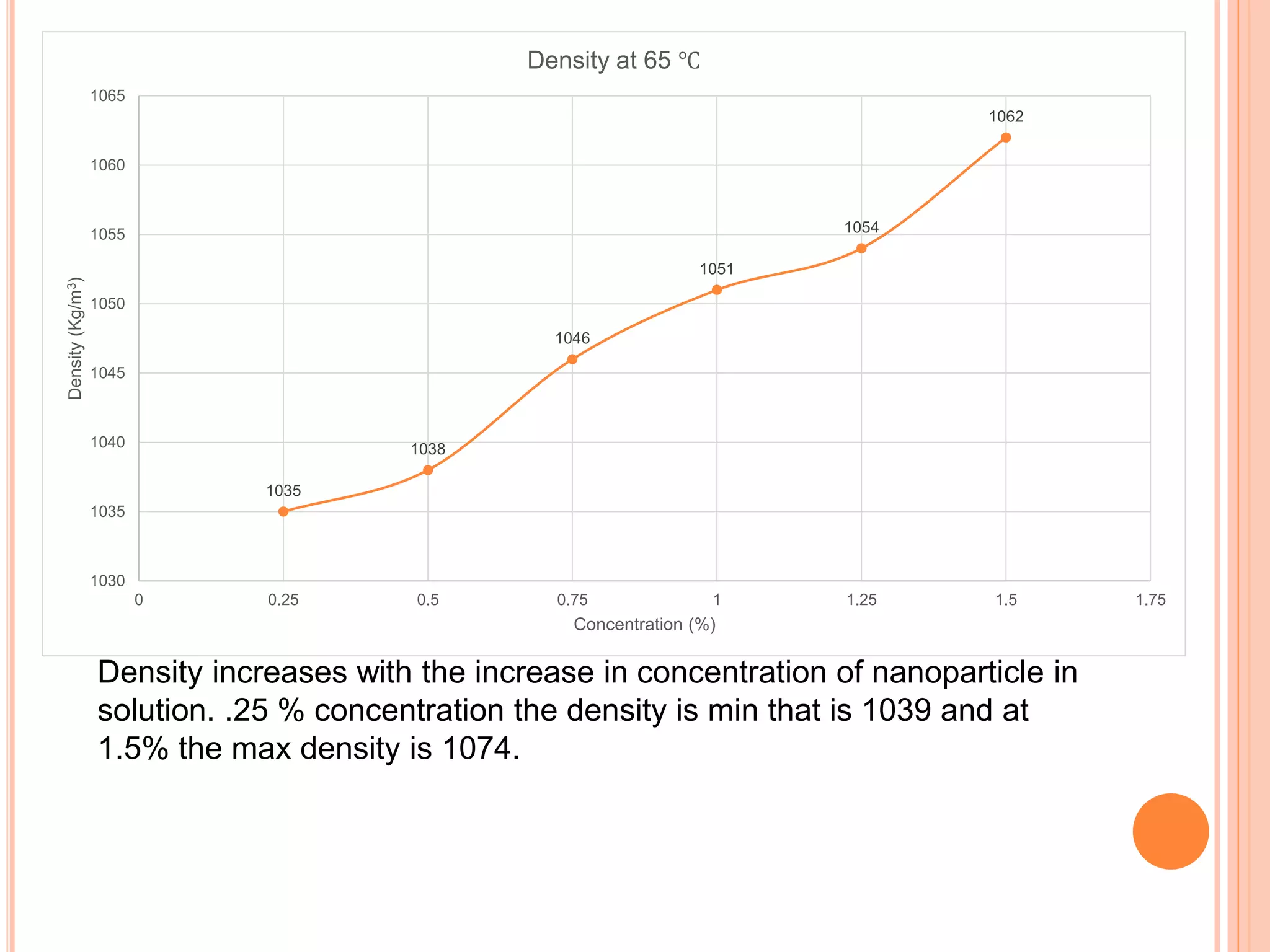
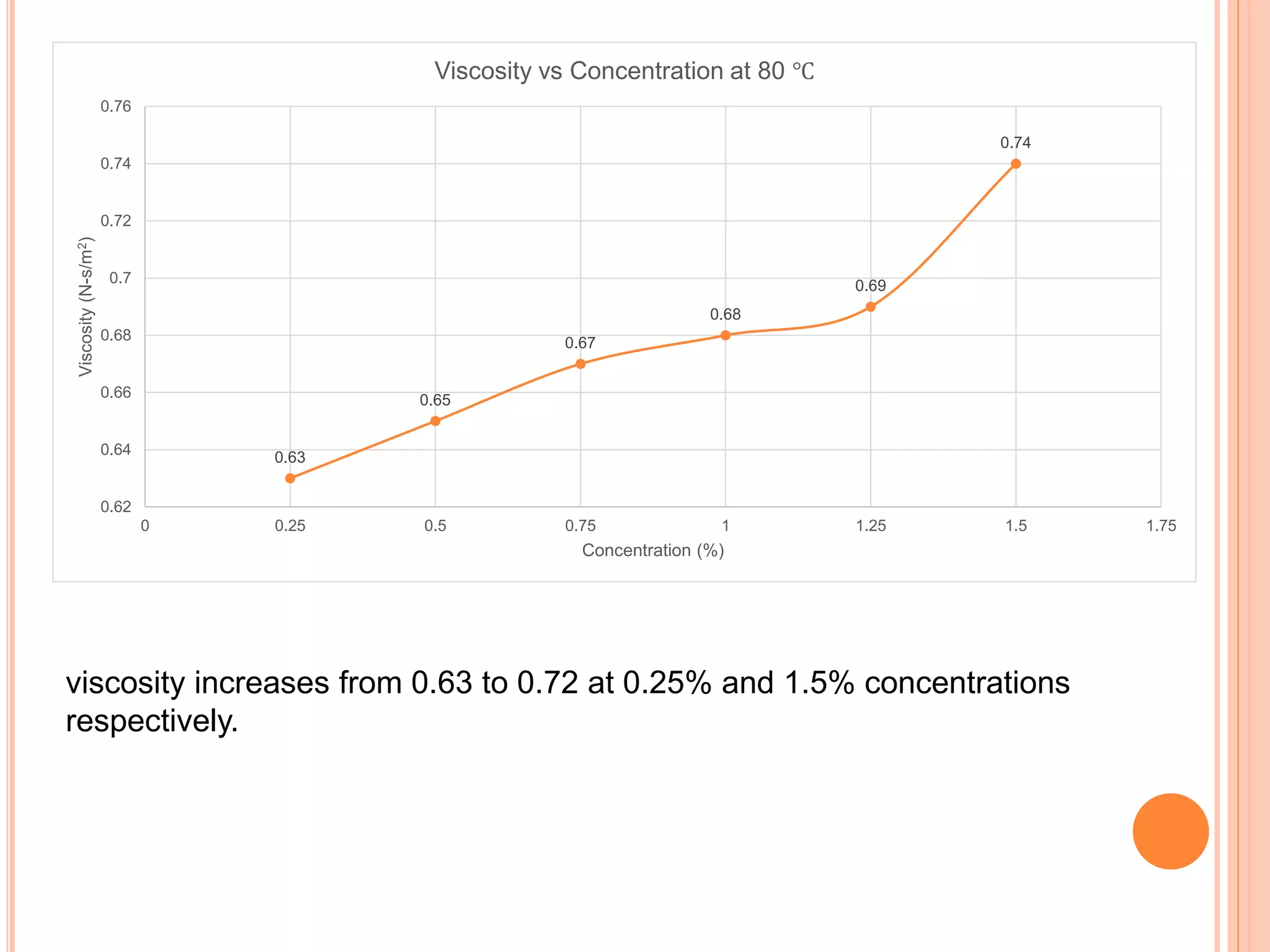
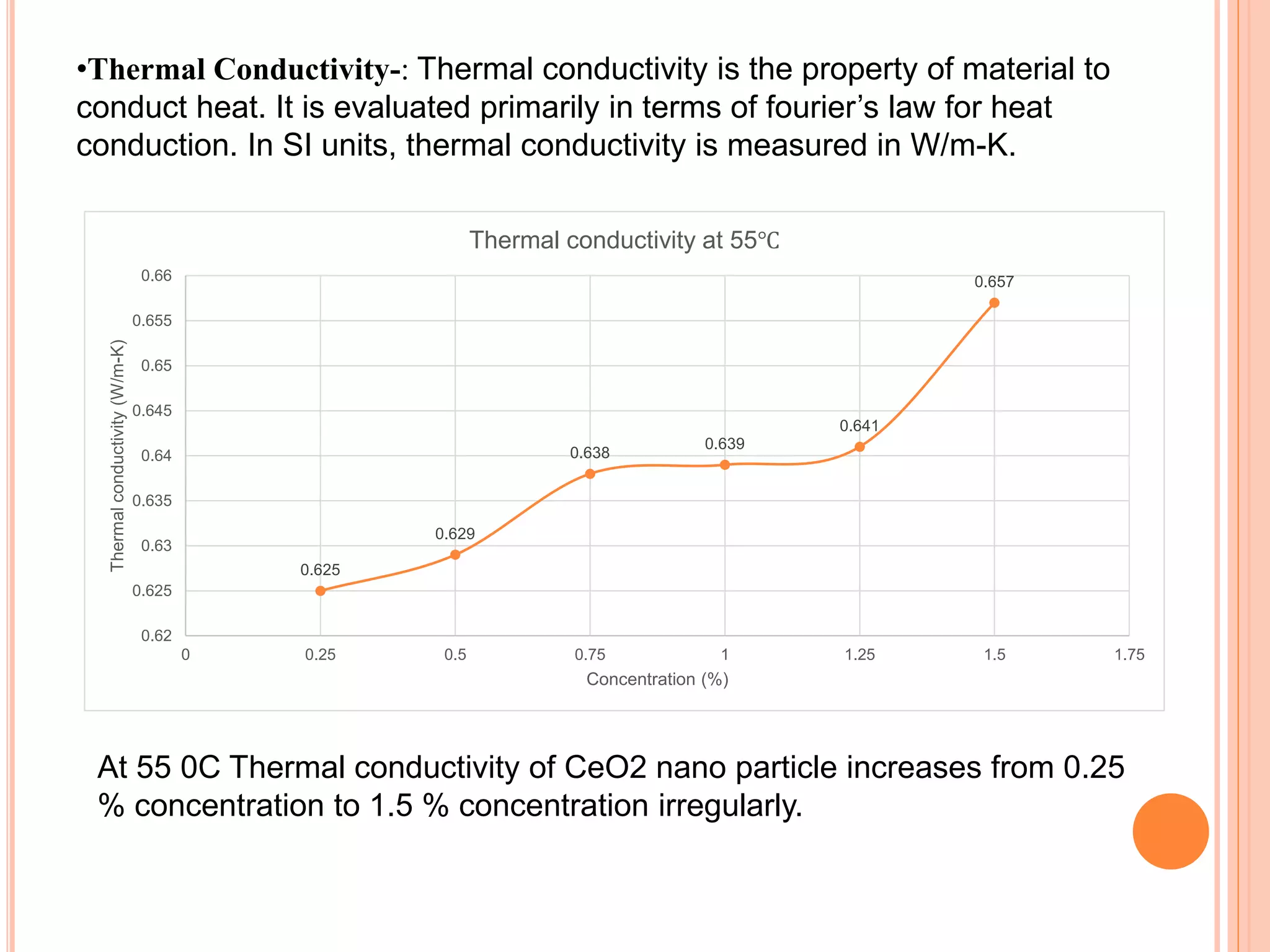
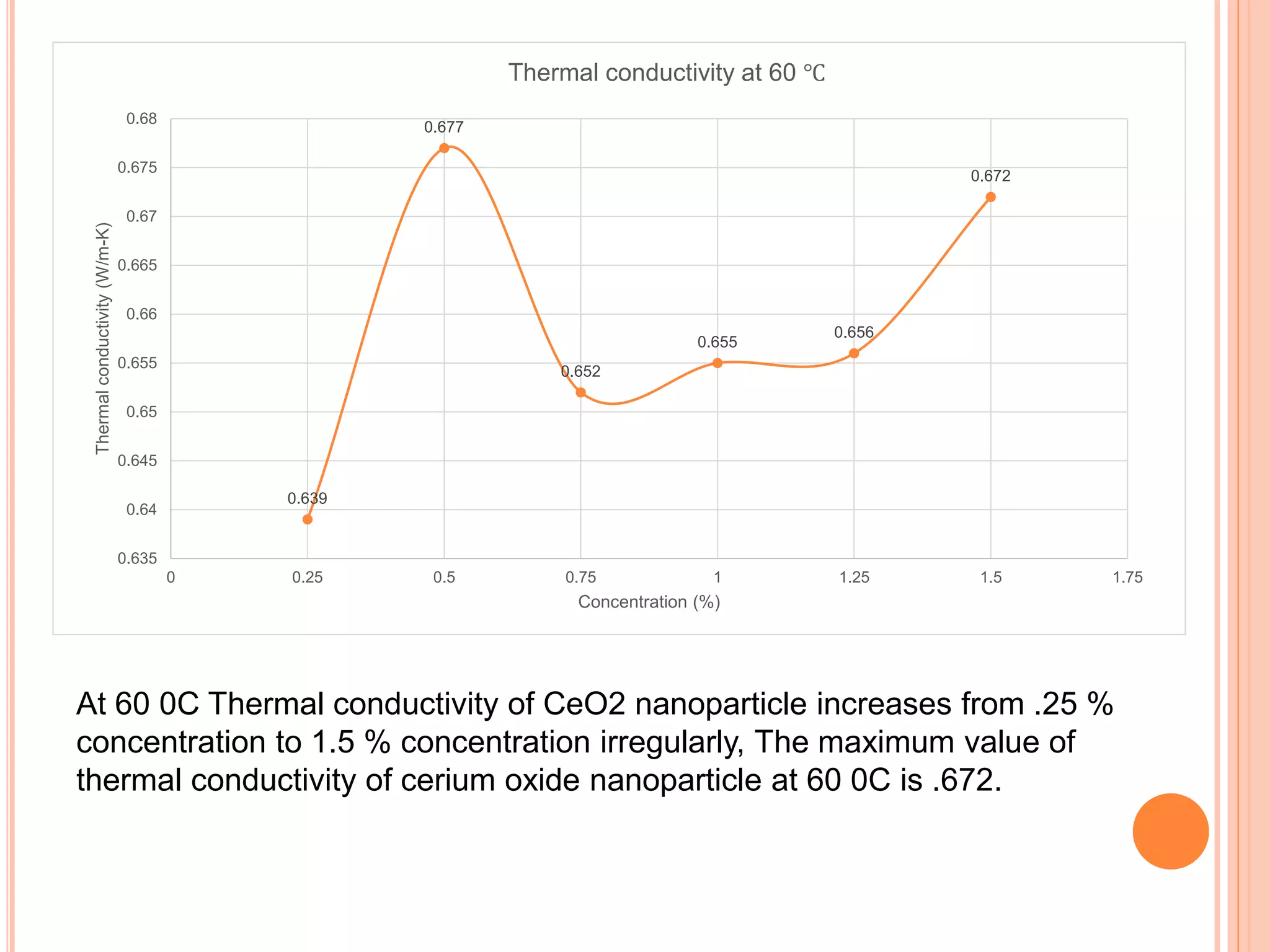
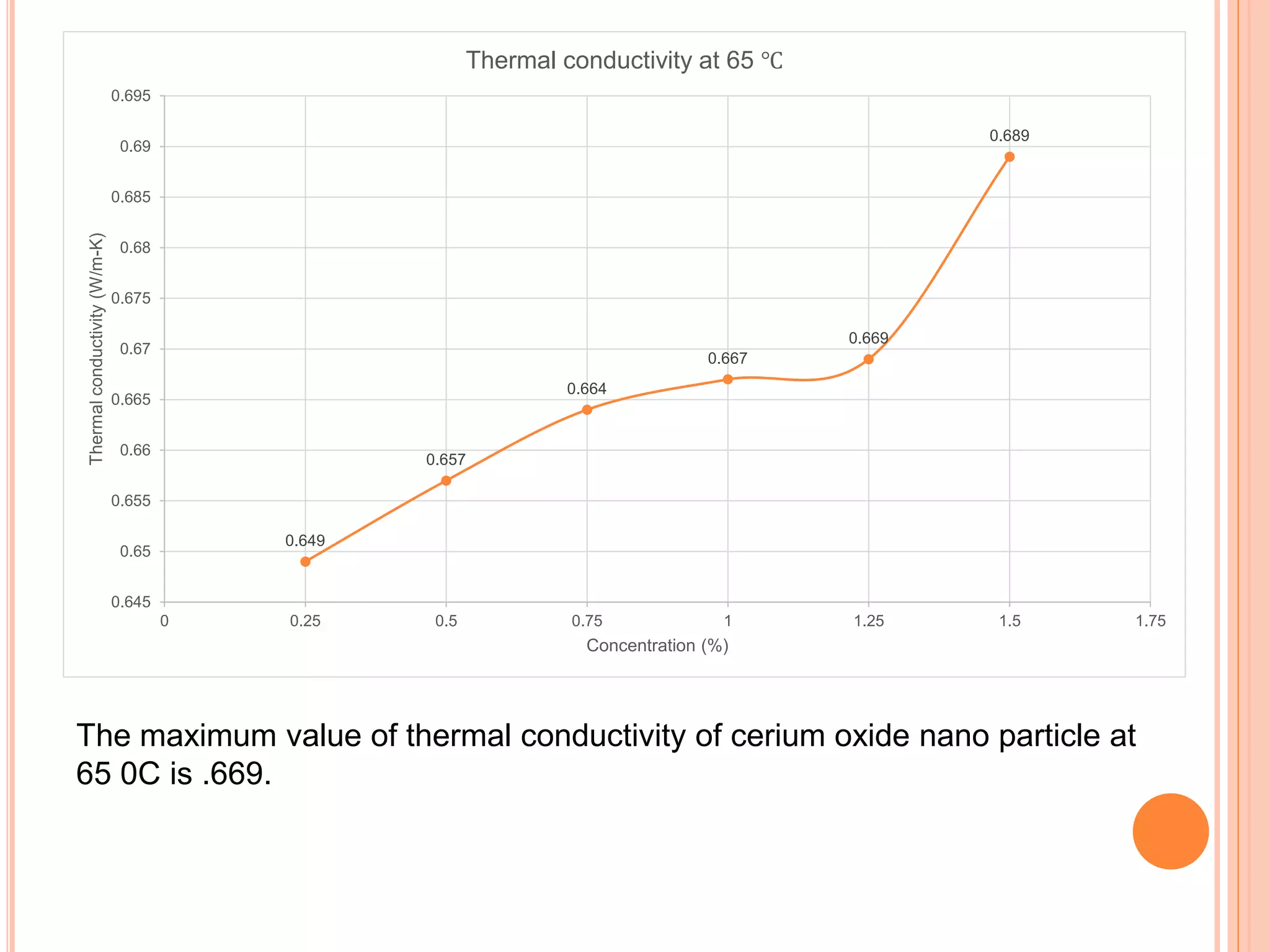
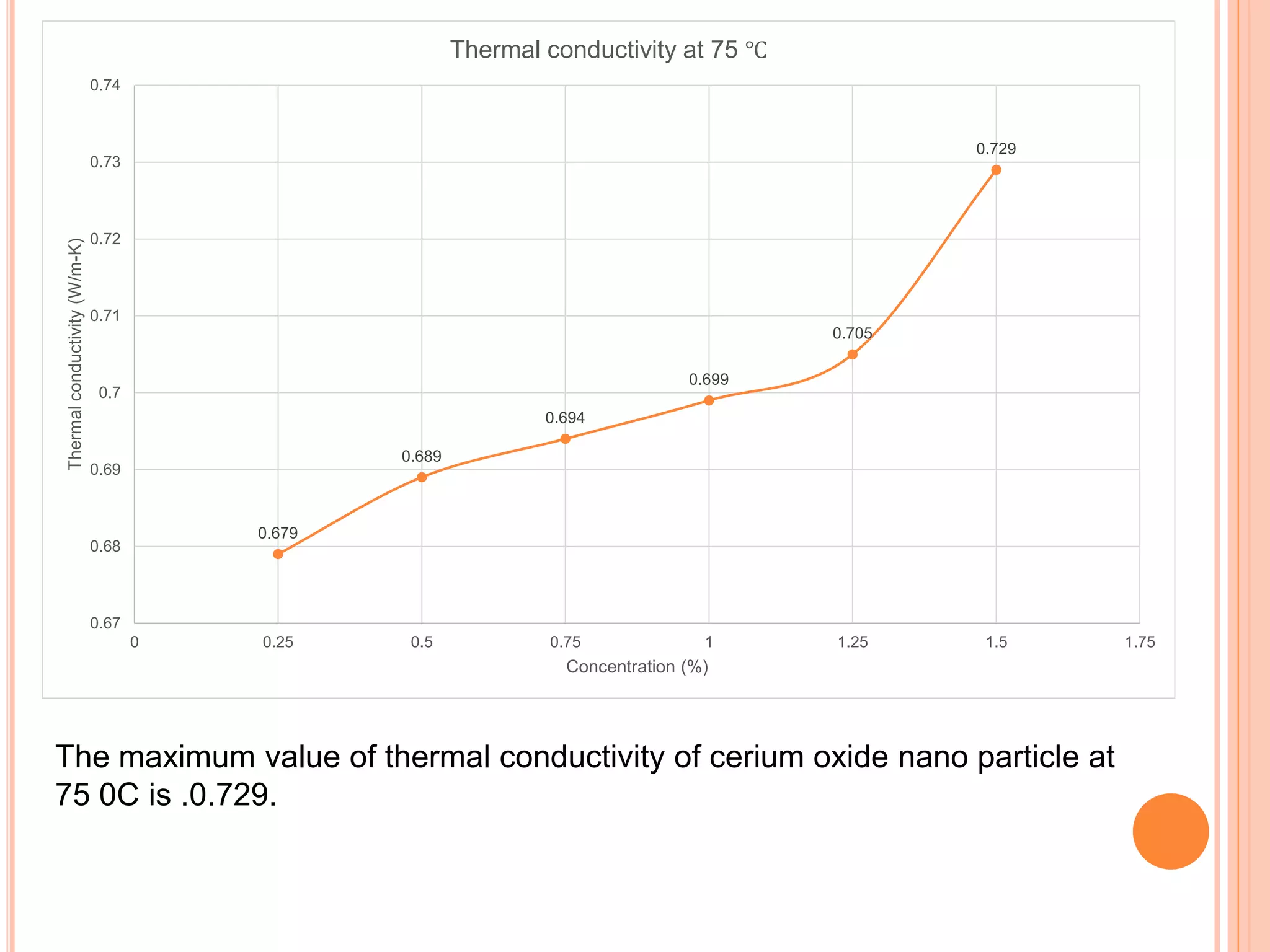
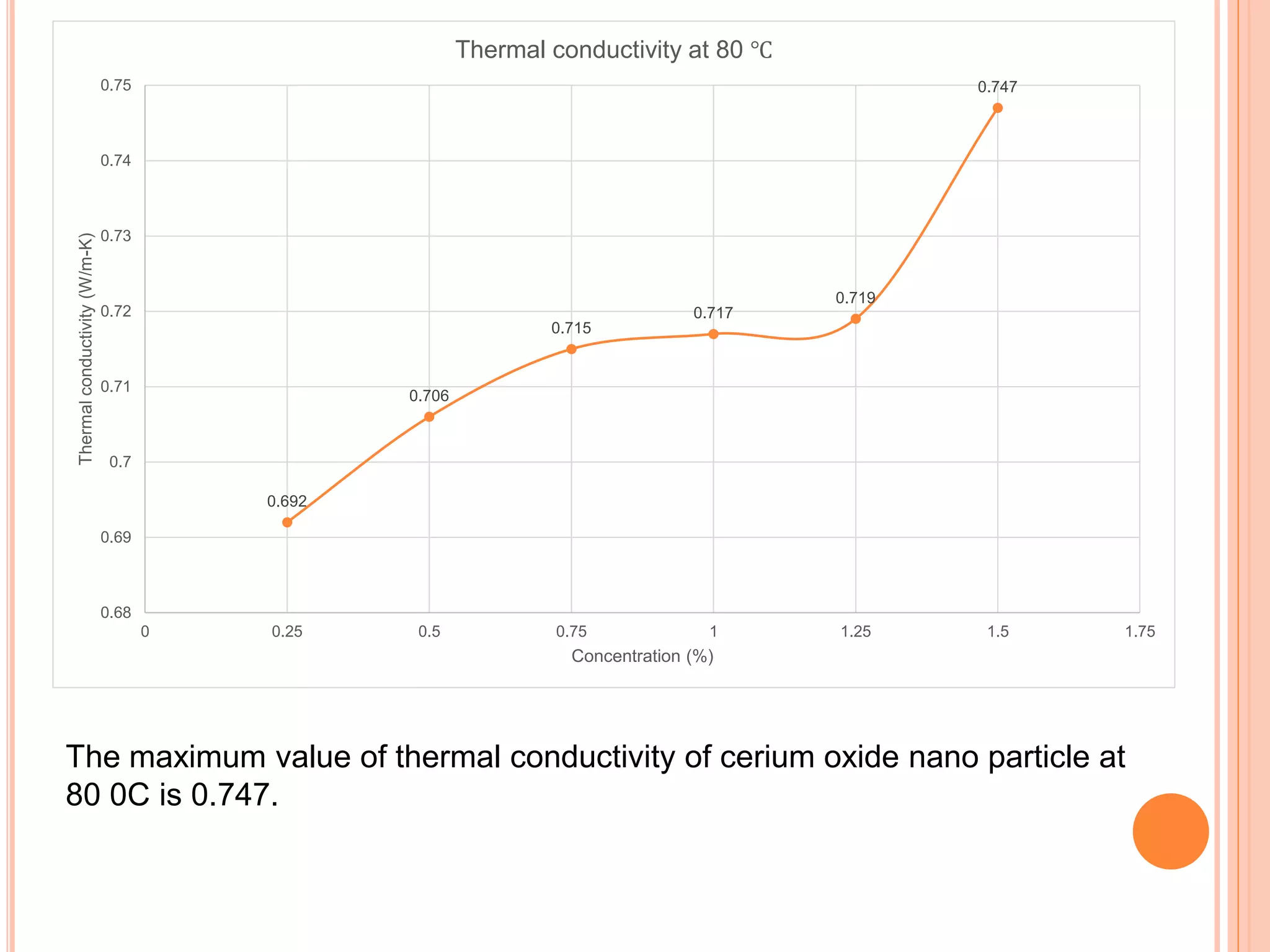
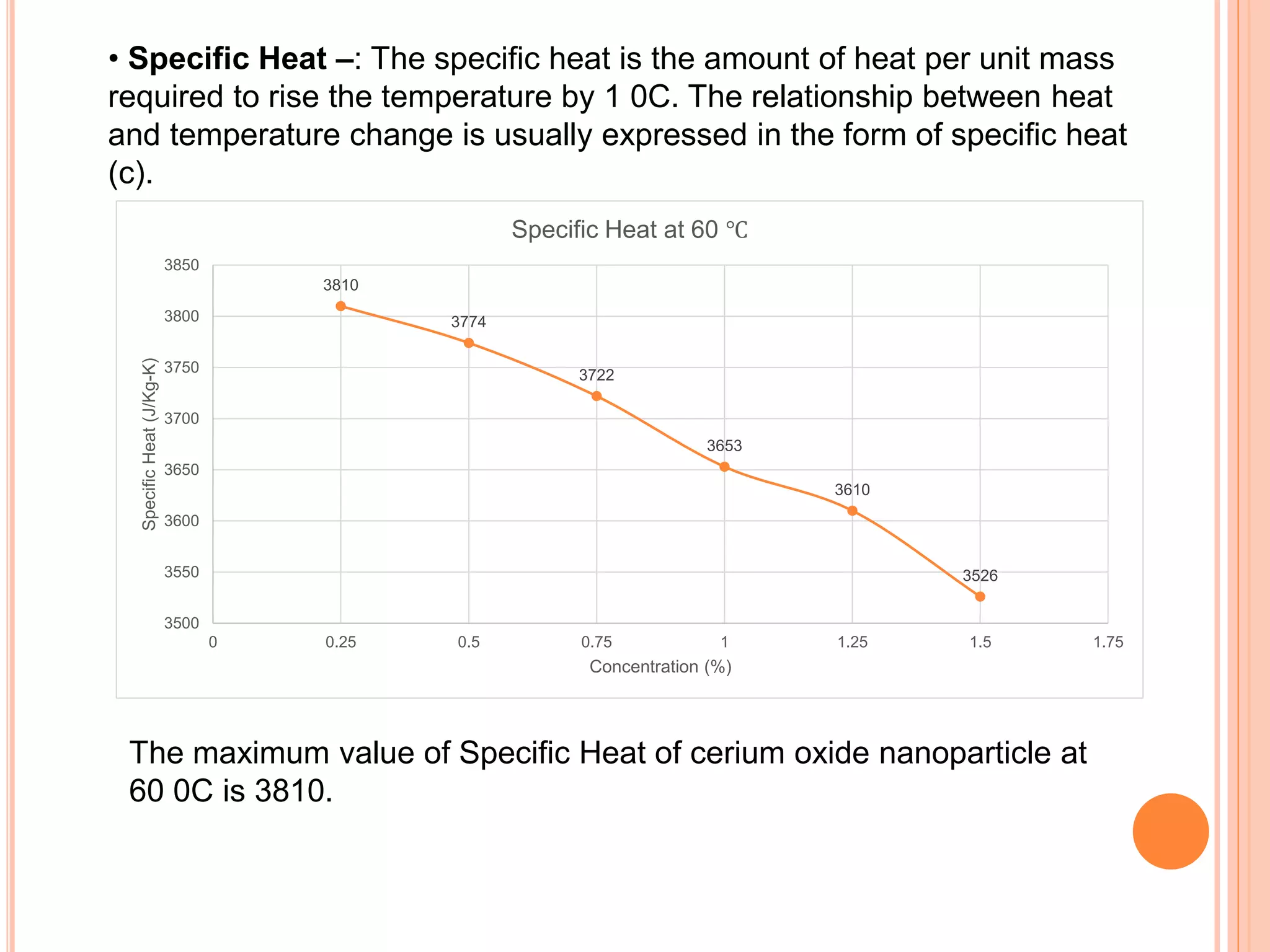
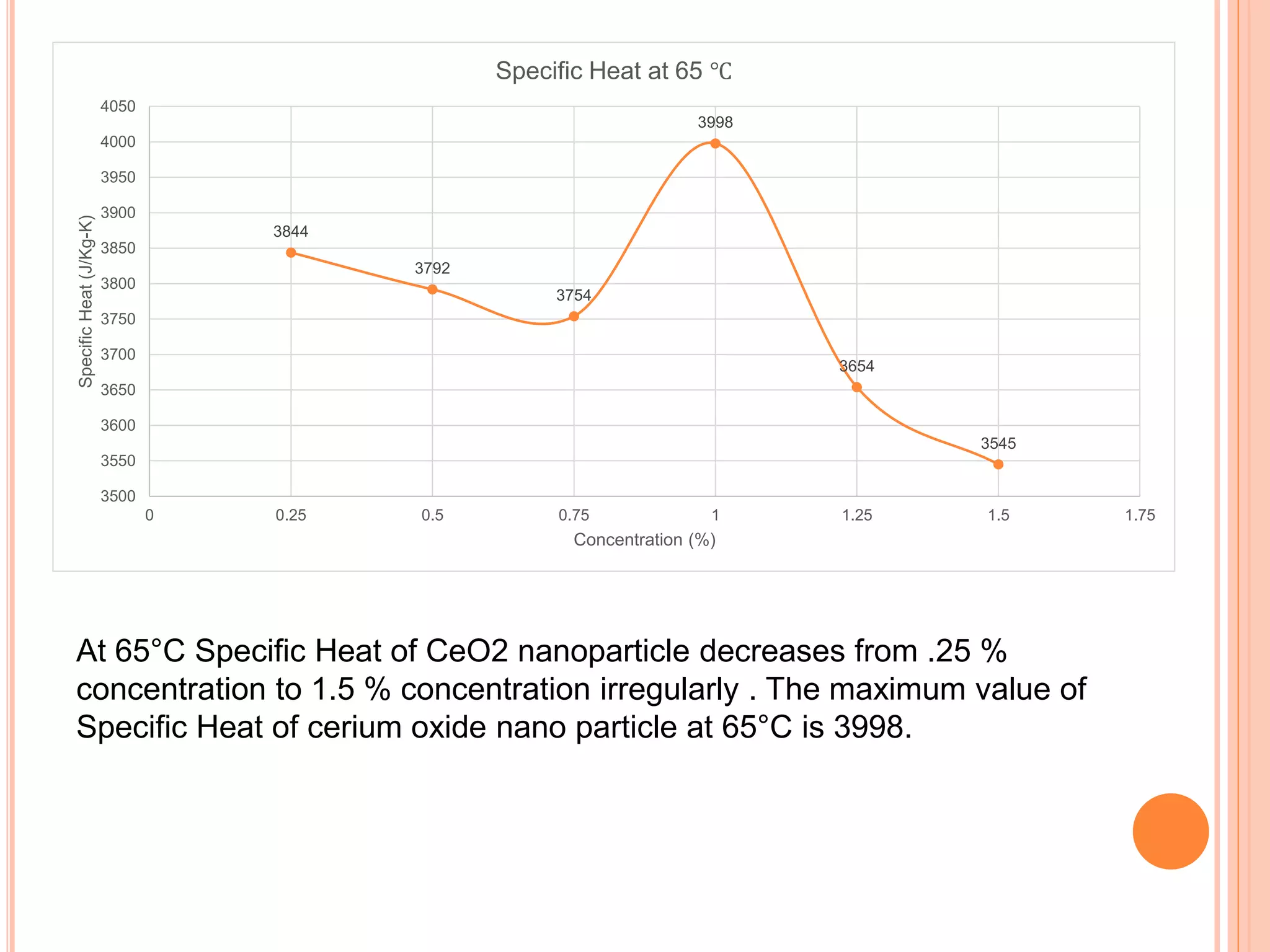
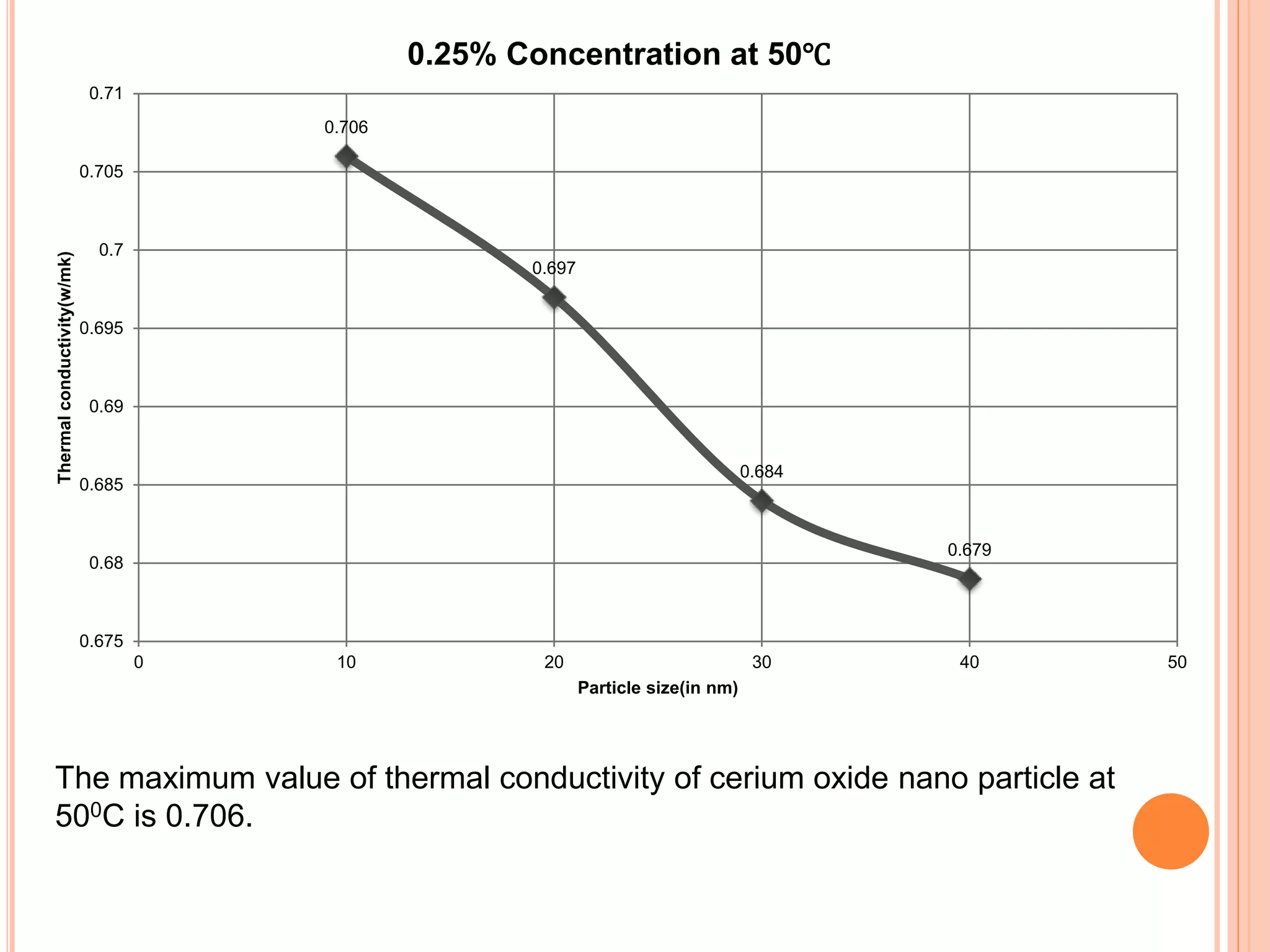
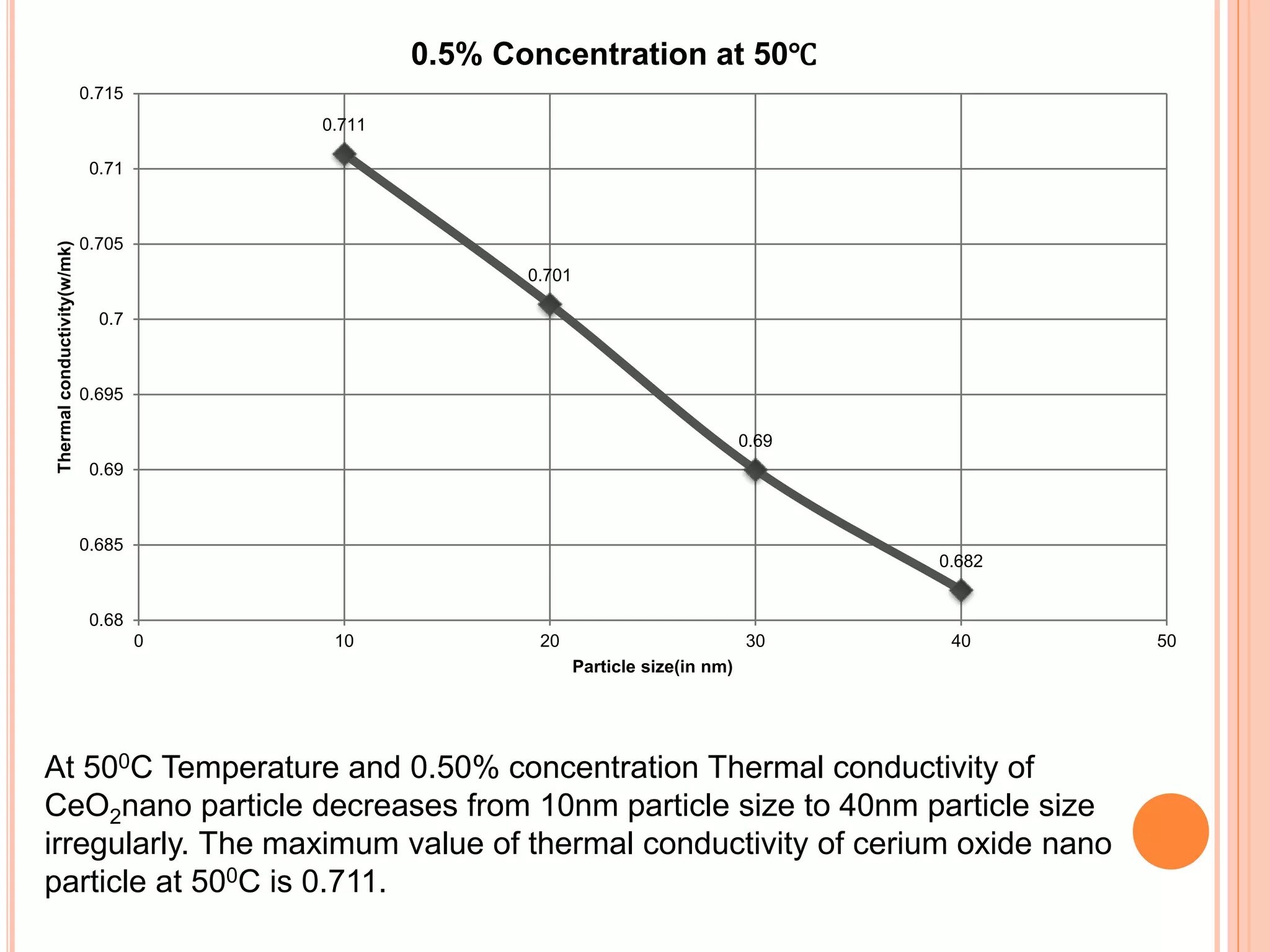
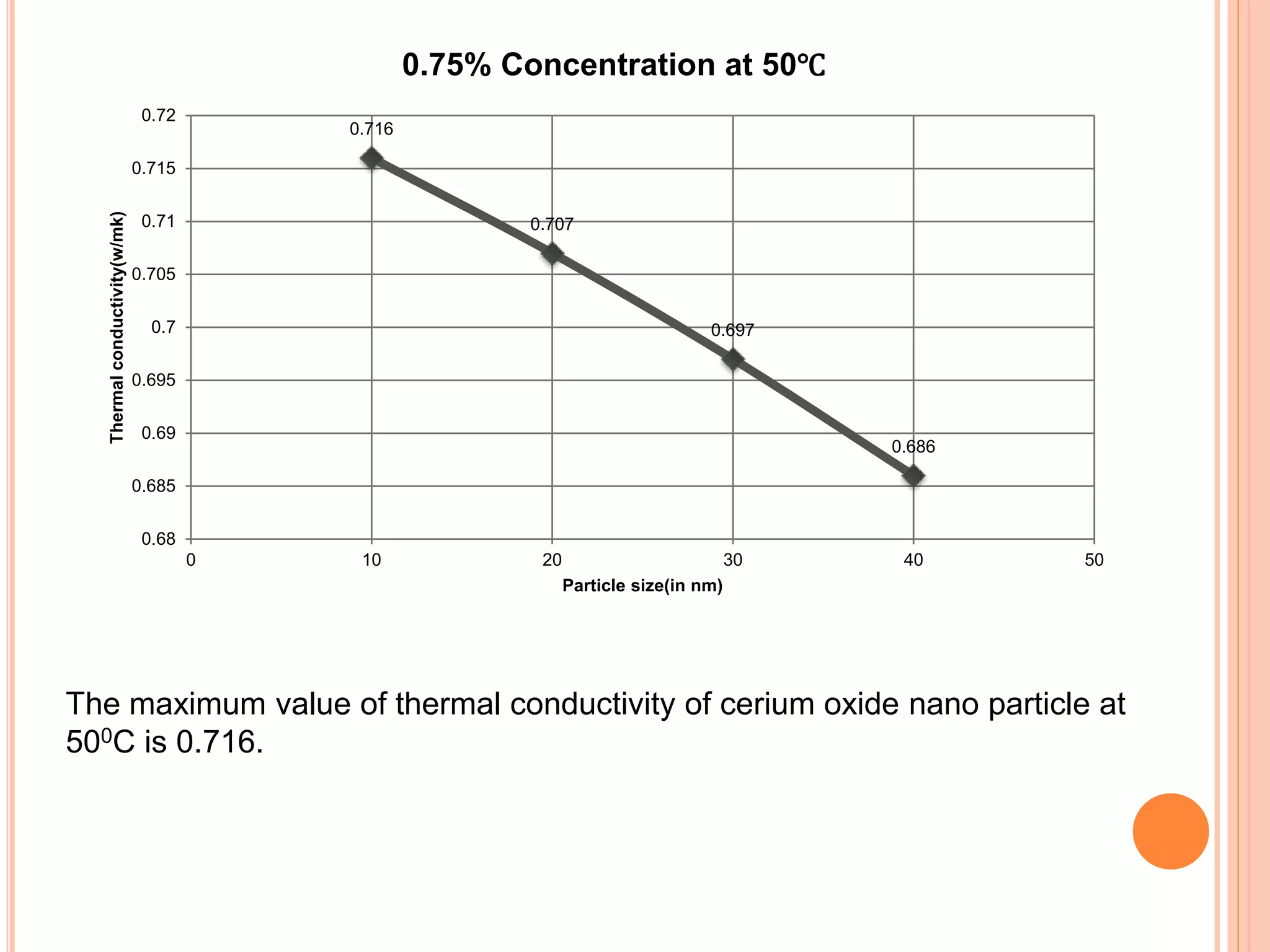
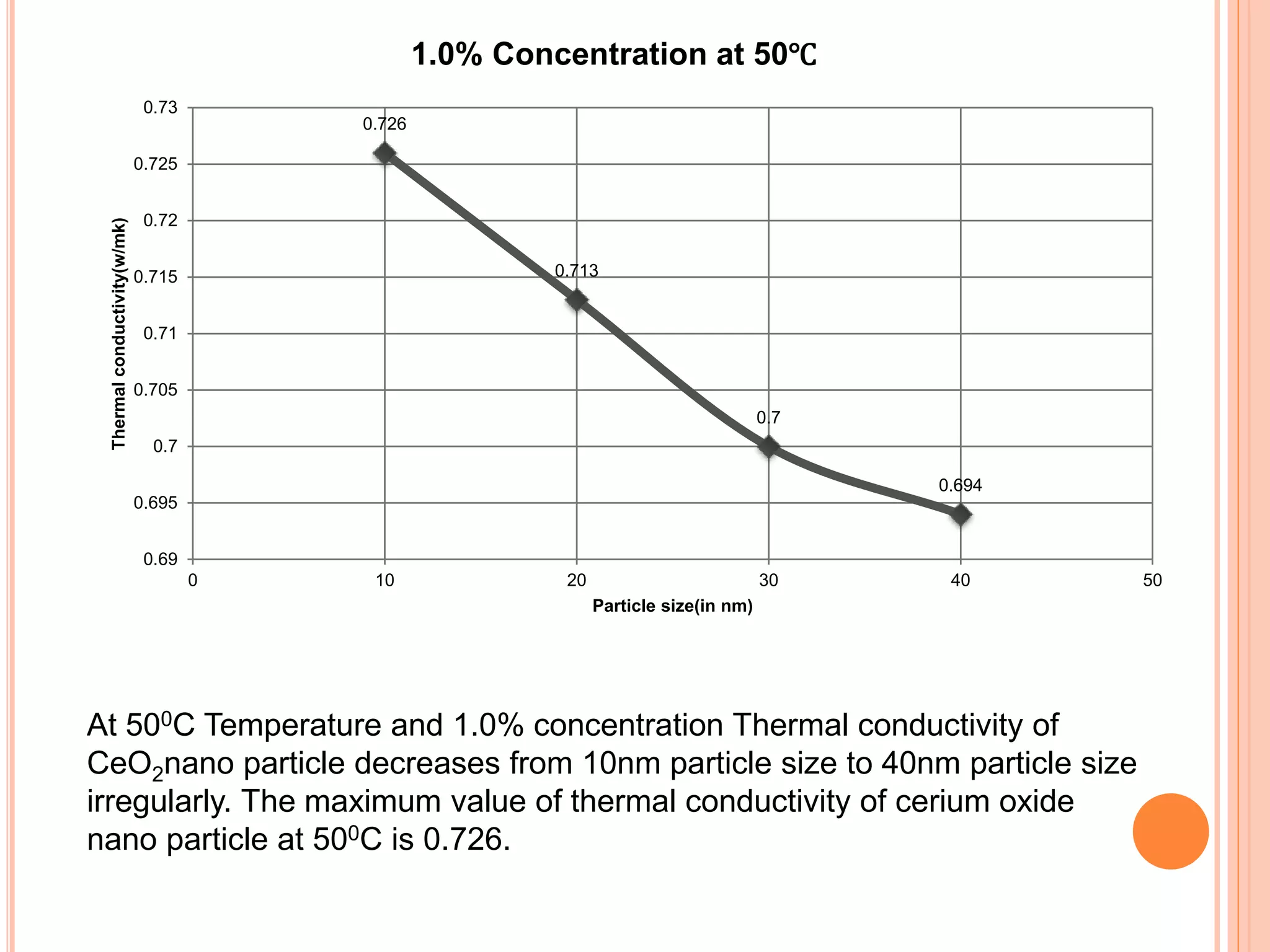
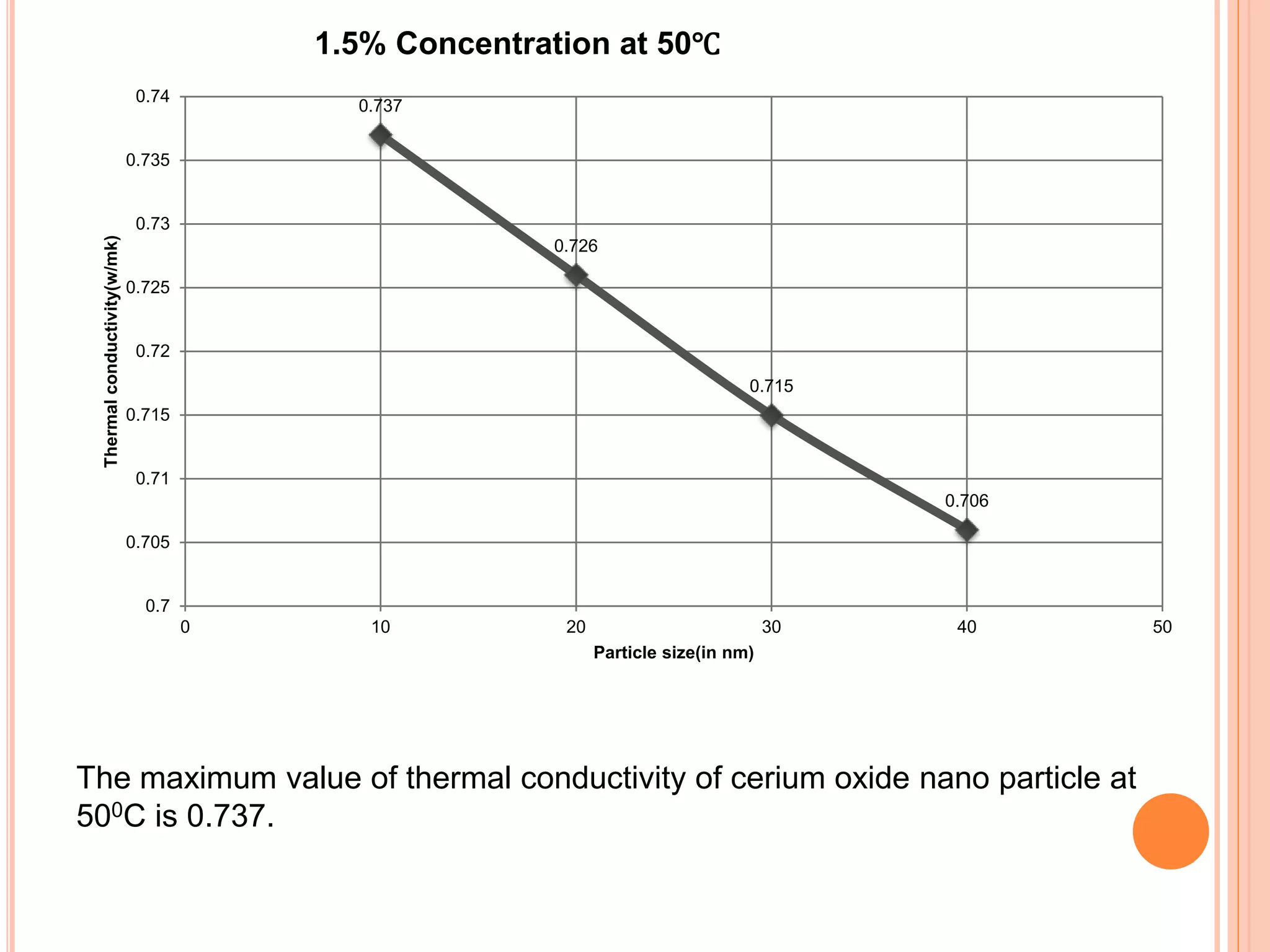
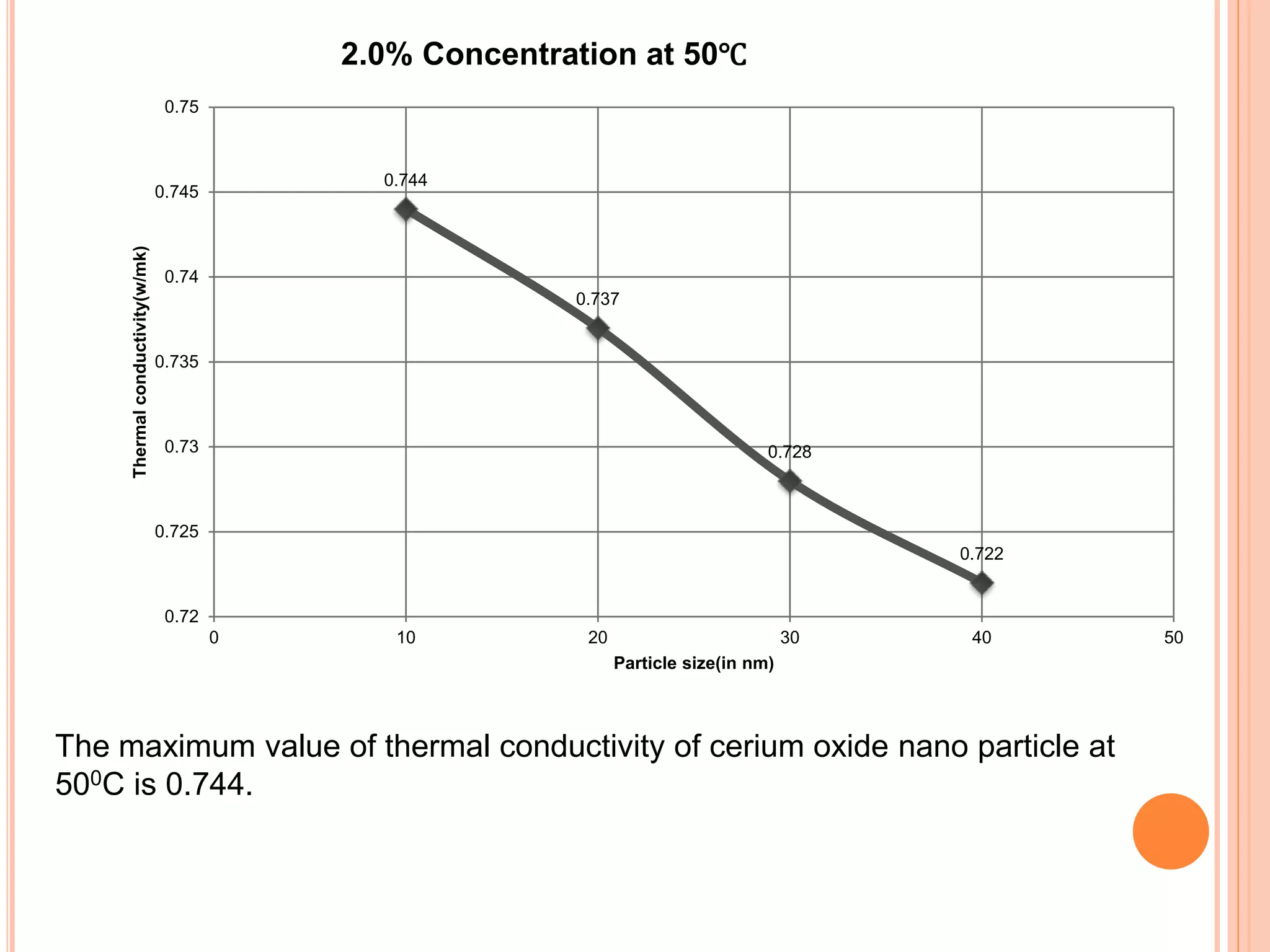
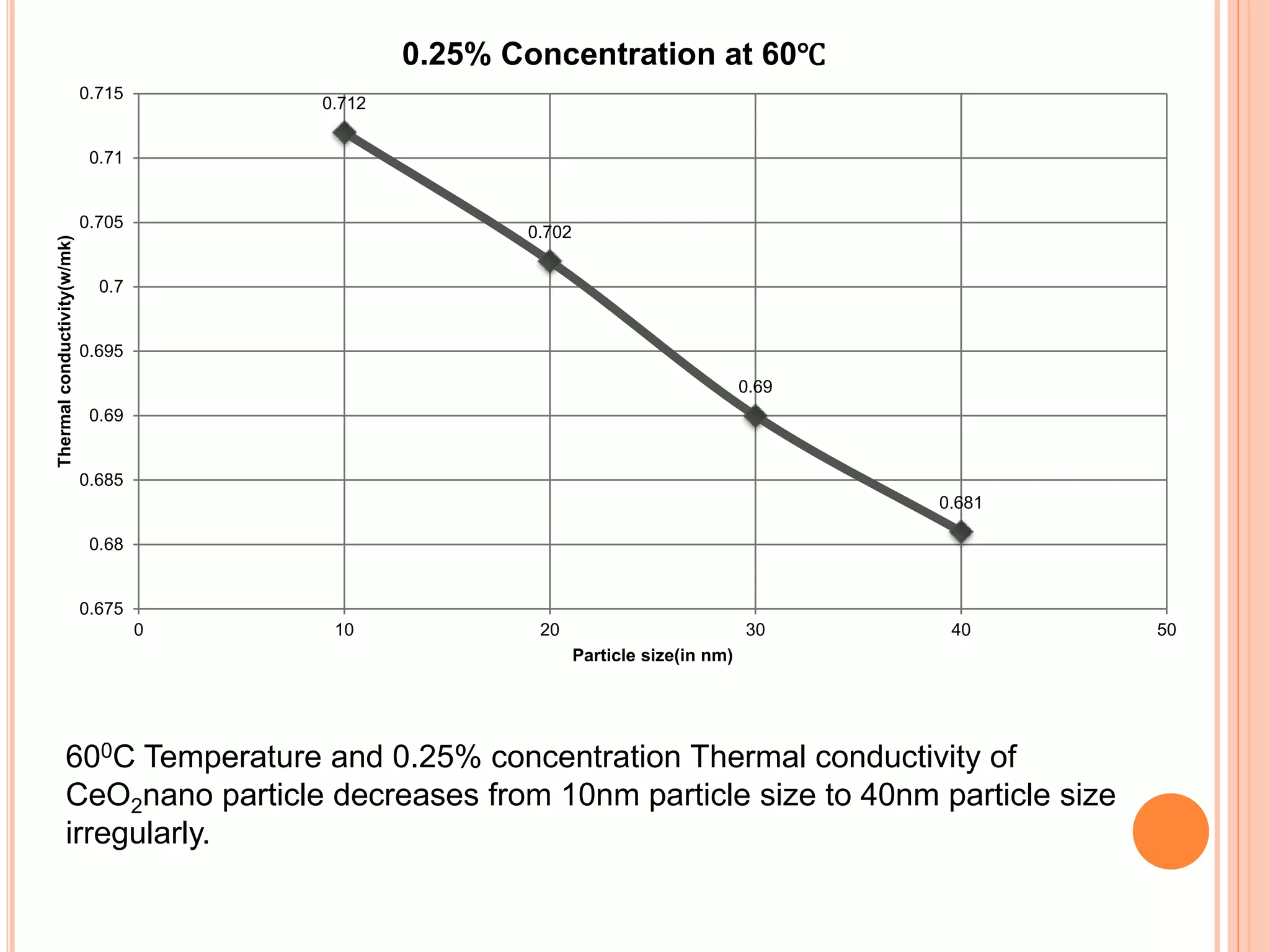
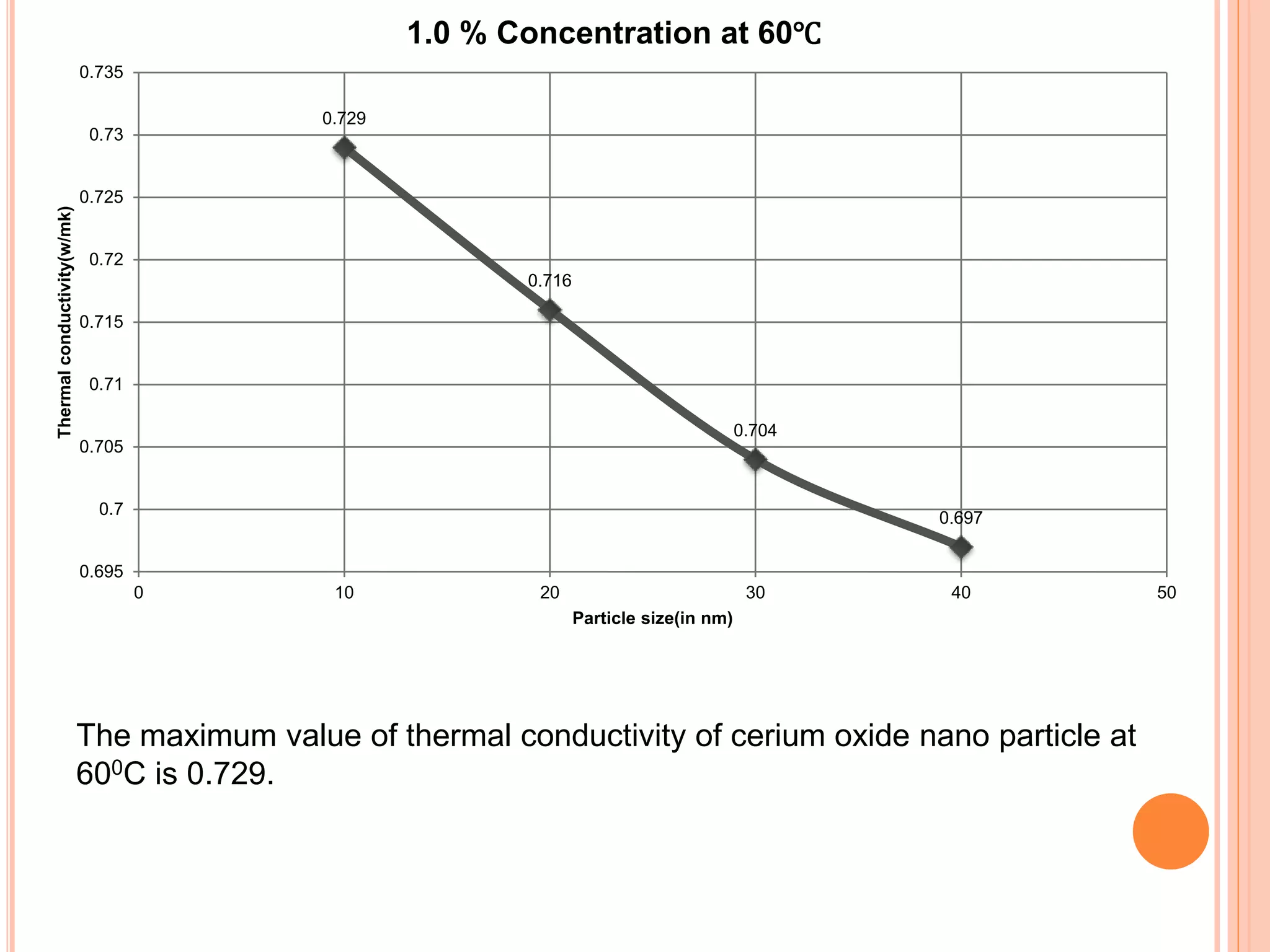
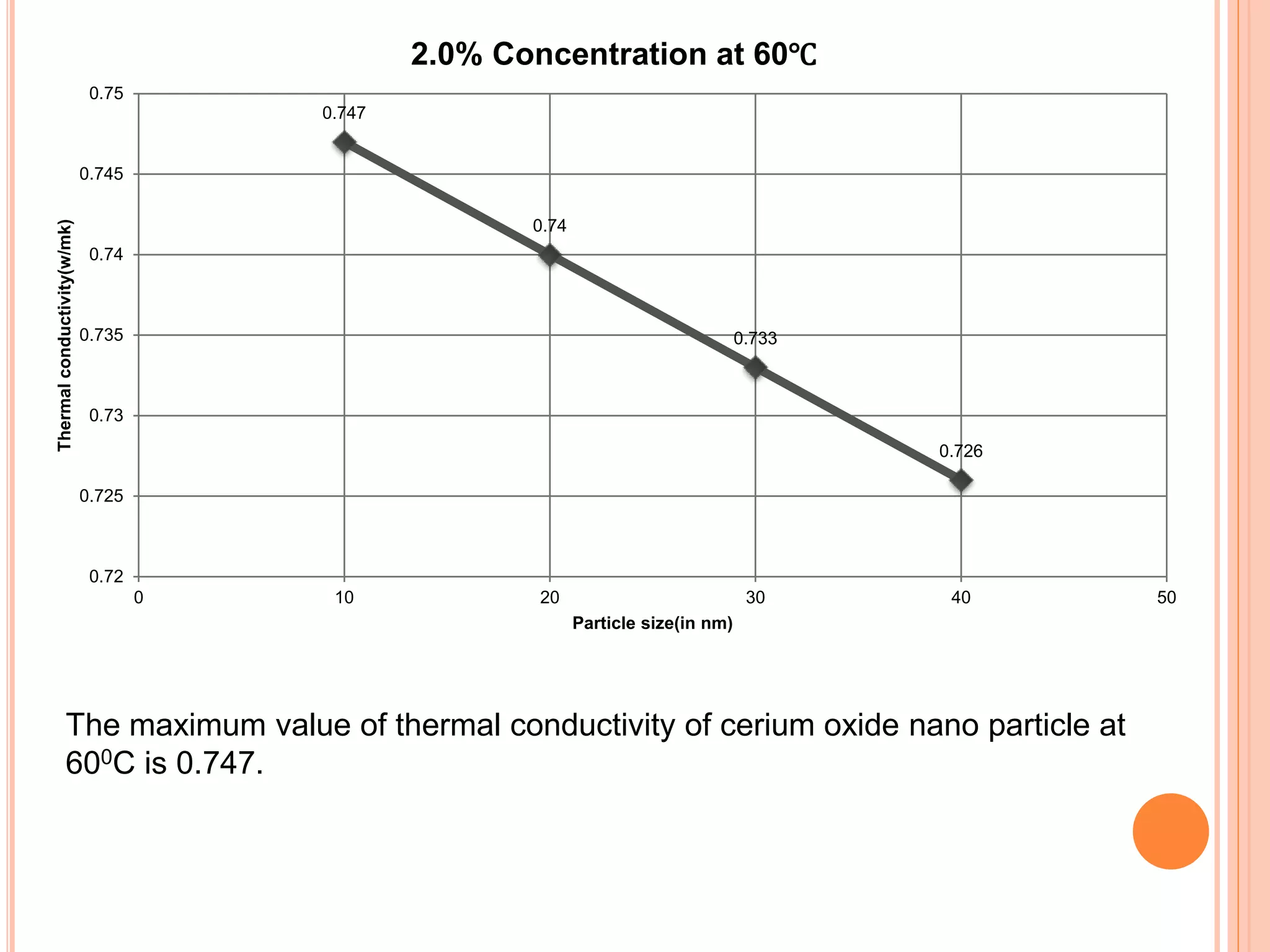
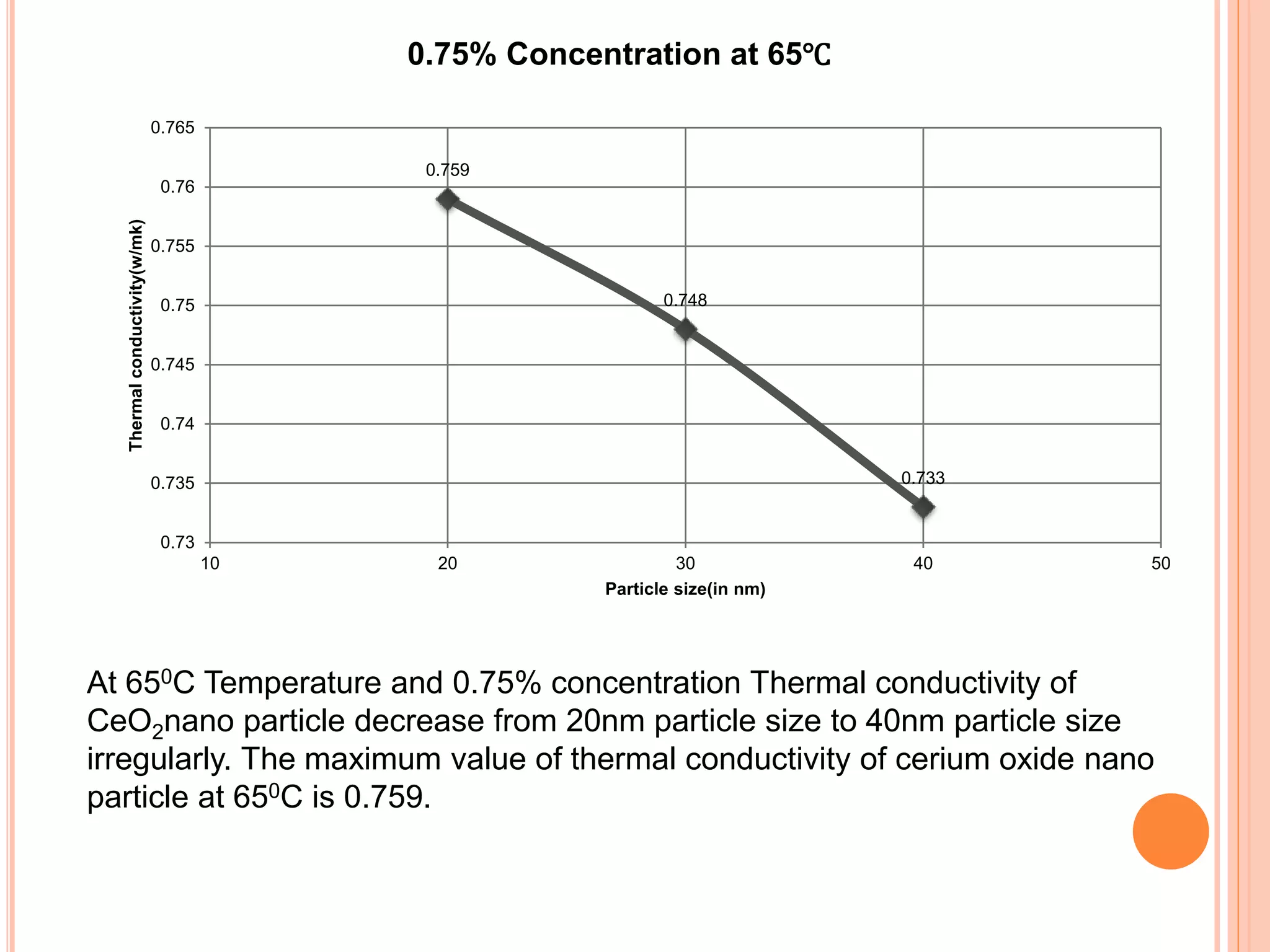
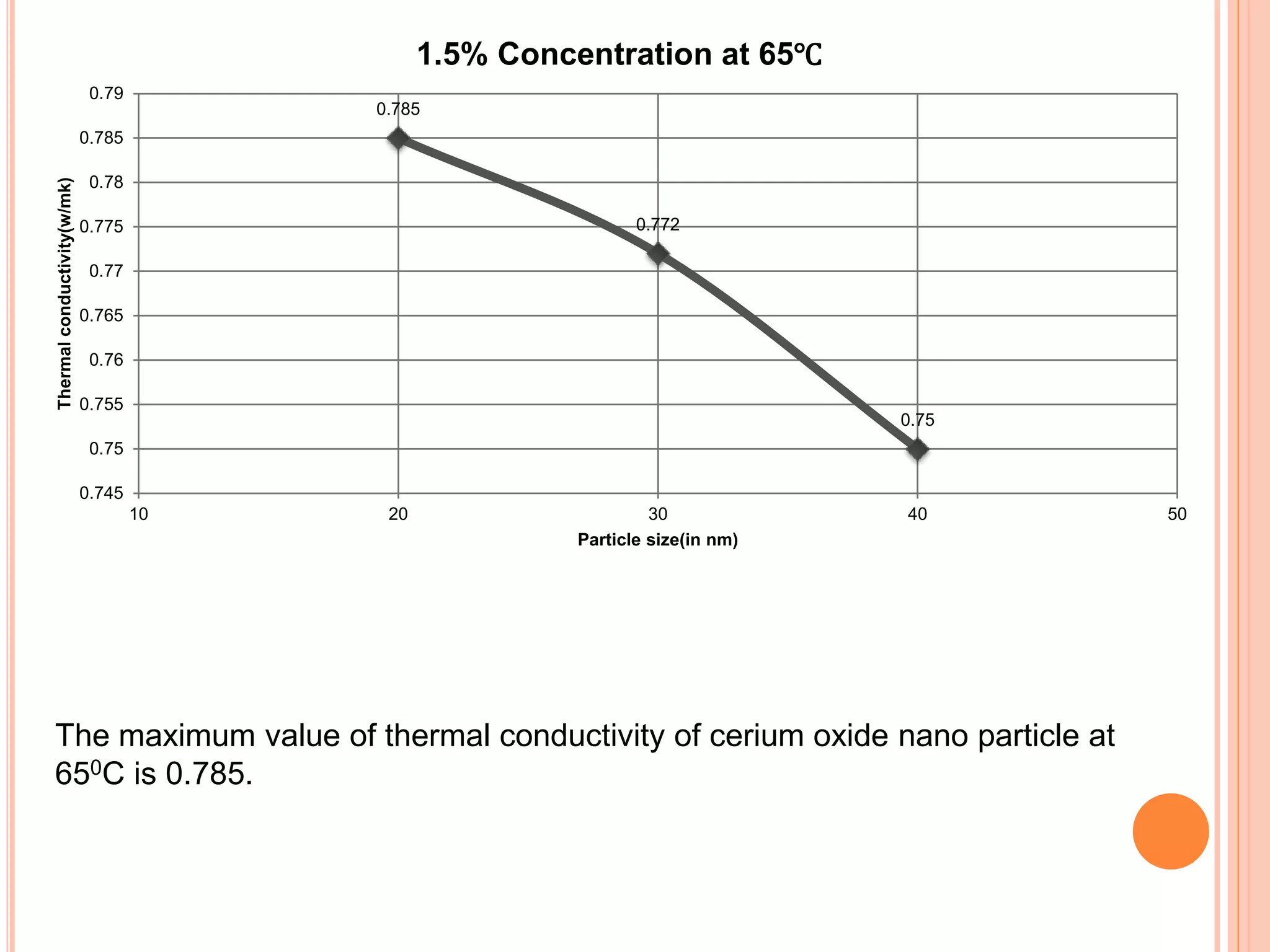
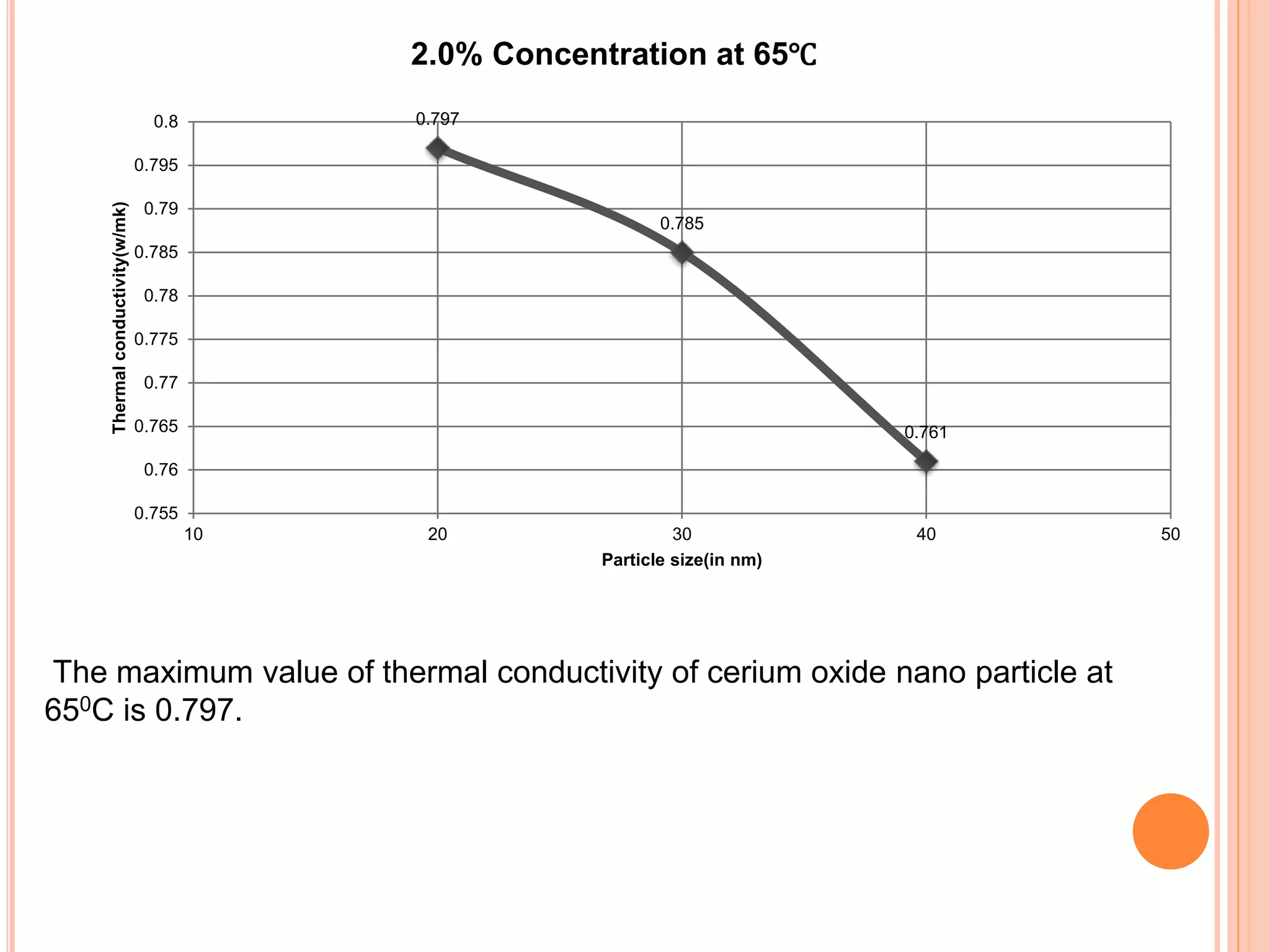
This document summarizes the synthesis and characterization of a nanofluid with water as the base fluid. It discusses the types of nanoparticles and nanofluids, describes the synthesis of cerium oxide nanoparticles and the nanofluid, and presents the experimental methodology used to measure properties like density, viscosity, and thermal conductivity of the nanofluid at varying temperatures and nanoparticle concentrations. The results show that the density, viscosity, and thermal conductivity of the nanofluid increase with increasing nanoparticle concentration. The maximum thermal conductivity achieved is 0.747 W/m-K at a concentration of 1.5% and temperature of 75°C.







![REFERENCES
[1]. Wisut Chamsa-ard,Sridevi Brundavanam,Chun Che Fung , Derek
Fawcett and Gerrard Poinern 1,” Nanofluid Types, Their Synthesis,
Properties and Incorporation in Direct Solar Thermal Collectors: A Review”,
Nanomaterials 2017, 7, 131
[2]. Tae Il Kim , Yong Hoon Jeong, Soon Heung Chang, An experimental
study on CHF enhancement in flow boiling using Al2O3 nano-fluid, T.I. Kim
et al. / International Journal of Heat and Mass Transfer 53 (2010) 1015–1022
[3]. A.A. Mohamad, Myth about nano-fluid heat transfer enhancement, A.A.
Mohamad / International Journal of Heat and Mass Transfer 86 (2015) 397–
403
[4]. G. Narendar, A.V.S.S Kumara Swami Gupta,A. Krishnaiah,Satyanarayana
M.G.V., Experimental investigation on the preparation and applications of
Nano fluids, G. Narendar et.al./ Materials Today: Proceedings 4 (2017) 3926–
3931.
[5]. Tun-Ping Teng, Li Lin, and Chao-Chieh Yu, Preparation and
Characterization of Carbon Nanofluids by Using a Revised Water-Assisted
Synthesis Method, Journal of Nanomaterials Volume 2013, Article ID 582304](https://image.slidesharecdn.com/projectppt-180611122313/75/ppt-on-characterization-and-synthesis-of-nanofluid-with-base-fluid-water-49-2048.jpg)
![[6].Ramakoteswara Rao N1 and Leena Gahane2, ULTRASONIC AND
THERMAL CONDUCTIVITY STUDY OF ZNO NANOFLUIDS, Volume: 02
Issue: 10 October – 2017 (IJRIER)
[7].B. Wang, X. Wang, W. Lou, and J. Hao, “Rheological and tribological
properties of ionic liquid-based nanofluids 16 Journal of
Nanomaterialscontaining functionalized multi-walled carbon nanotubes,”
Journal of Physical Chemistry C, vol. 114, no. 19, pp. 8749–8754, 2010.
[8].Ved Prakash, Vinay Kumar Tyagi and Ajay Kumar Tyagi,Thermal
Conductivity and Dispersion Stability of Copper Oxide Nanofluid in
Kerosene,Ved Prakash, et al., Nano Vision, Vol.6 (2), 10-17 (2016)
[9].Murshed, S.M.S., Milanova, D., Kumar, R., 2009. An experimental study
of surface tension-dependent pool boiling characteristics of carbon
nanotubes-nanofluids. In: ASME 2009 7th International Conference on
Nanochannels, Microchannels, and Minichannels. American Society of
Mechanical Engineers, pp. 75e80
[10].Jure Ravnik, MatjazHriberšek, L. Škerget, Analysis of three-dimensional
natural convection of nanofluids by BEM, DOI:
10.1016/j.enganabound.2010.06.019
[11].Eiyad Abu-Nada, Effects of Variable Viscosity and Thermal Conductivity
of CuO-Water Nanofluid on Heat Transfer Enhancement in Natural
Convection: Mathematical Model and Simulation, Journal of Heat Transfer
132(5):052401 · May 2010](https://image.slidesharecdn.com/projectppt-180611122313/75/ppt-on-characterization-and-synthesis-of-nanofluid-with-base-fluid-water-50-2048.jpg)