Embed presentation
Downloaded 13 times






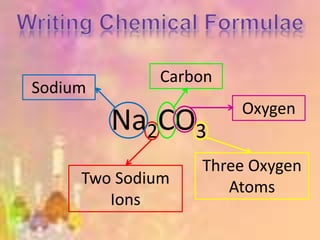





This document provides information on naming and writing formulas for ionic compounds: - Ions are atoms or groups of atoms that have gained or lost electrons, giving them a positive or negative charge. Common ions and their charges should be memorized. - Prefixes indicate the number of atoms in polyatomic ions or in compound names. - To name ionic compounds, the cation (positively charged ion) is named first followed by the anion (negatively charged ion). Transition metals use Stock notation to indicate charge. - To write formulas, the charges of ions are used to balance the total charge of the compound to be neutral, choosing ions that satisfy the smallest whole number ratio.










