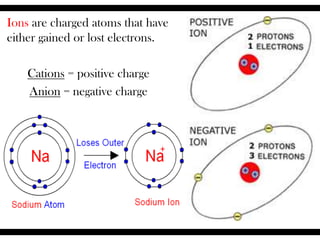
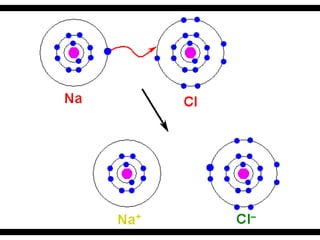
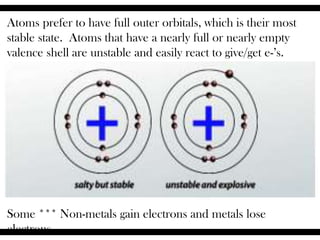
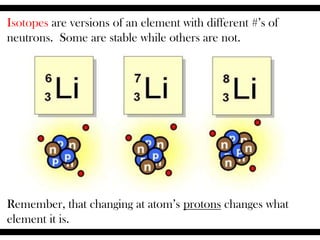
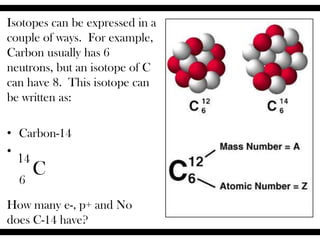
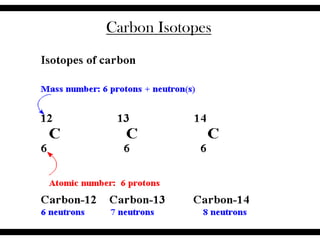
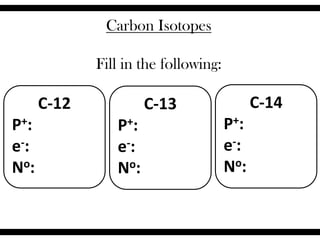
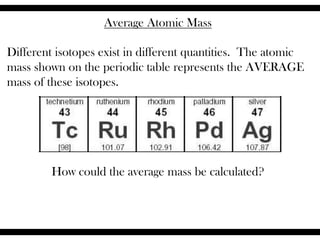
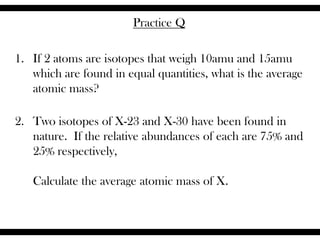
Ions are atoms that have gained or lost electrons to become charged. Cations have a positive charge from losing electrons while anions have a negative charge from gaining electrons. Atoms form stable ions by gaining or losing electrons to achieve a full outer electron shell. Isotopes are versions of an element with different numbers of neutrons. Some isotopes are stable while others are unstable. Isotopes can be written with the element name and mass number, such as Carbon-14. The average atomic mass shown on the periodic table is calculated from the relative abundances of naturally occurring isotopes.