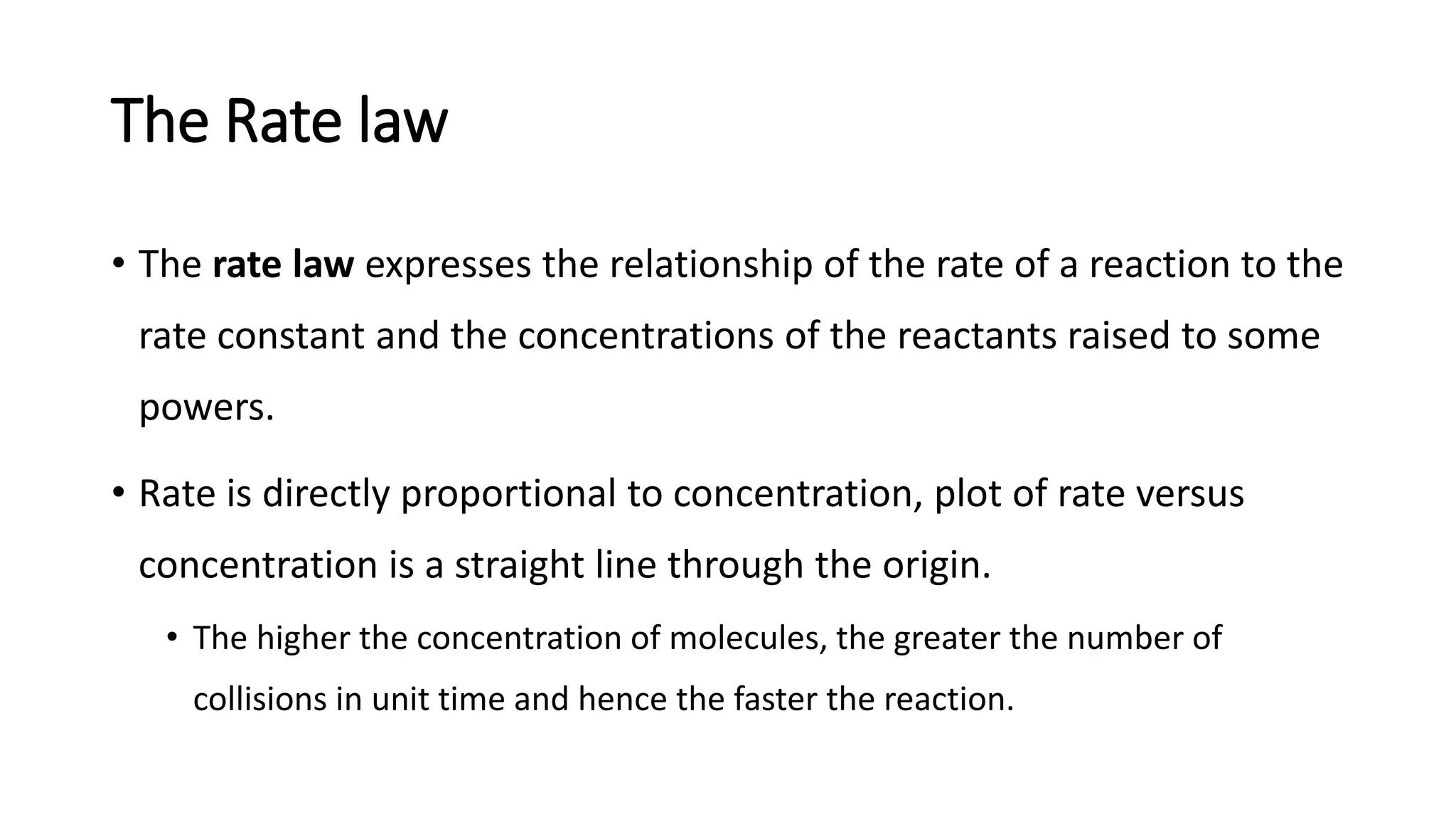
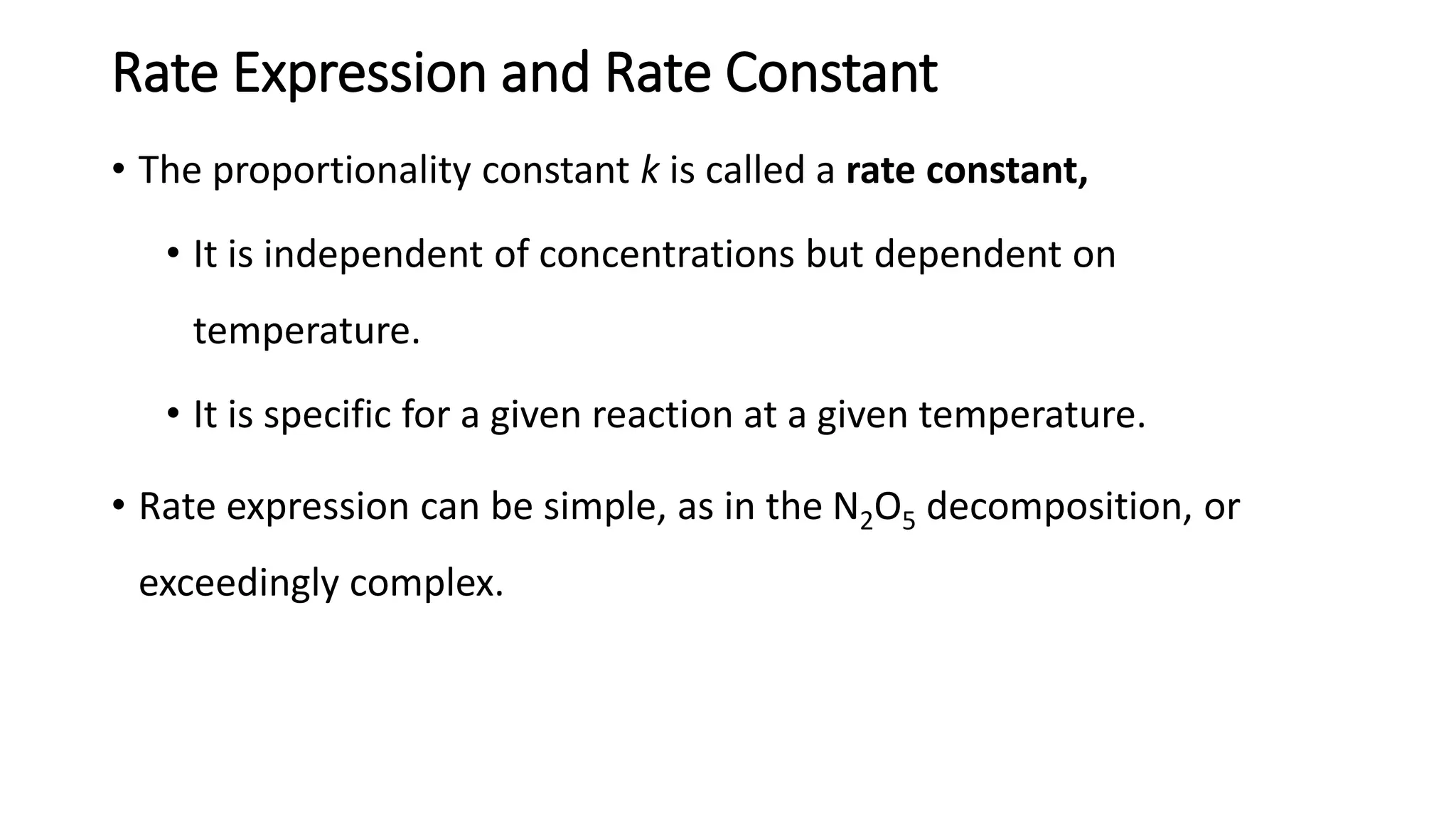
La cinética química estudia la velocidad de las reacciones químicas, centrándose en la tasa de consumo de reactivos y formación de productos, así como la influencia de condiciones de reacción. Se define la tasa de reacción como el cambio en la concentración de reactivos o productos por unidad de tiempo, y se utiliza la ley de la velocidad para relacionar estas tasas con las concentraciones de reactivos. Se determina el orden de reacción a partir de datos experimentales y puede aceptarse un orden fraccional en algunos casos.

![Reaction Rate
• The concentrations of NO2 and
O2 increase with time, whereas
that of N2O5 decreases. The
reaction rate is defined as
−[N2O5]
𝑡
=
NO2
2𝑡
=
[O2]
1
2
𝑡](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-3-2048.jpg)
![Expressing the Reaction Rate
• Reaction rate is measured in terms of the changes in
concentrations of reactants or products per unit time.
• For the general reaction aA +bB → cC + dD, where A,
B, C, and D represent substances in the gas phase (g)
or in aqueous solution (aq), and a, b, c, d are their
coefficients in the balanced equation
• We measure the concentration of A at t1 and at t2:
The negative sign is used because the concentration of A
is decreasing. This gives the rate a positive value.
Rate =
change in concentration of A
change in time
[A2]- [A1]
t2 - t1
= - =
−[𝐴]
𝑎𝑡
=
− B
𝑏𝑡
=
[𝐶]
𝑐𝑡
=
[𝐷]
𝑑𝑡](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-4-2048.jpg)


![Rate Expression and Rate Constant
• For the decomposition of N2O5:
Rate = = k[N2O5],
• This equation is referred to as the rate expression for the
decomposition of N2O5.
• It tells how the rate of the reaction N2O5 (g) → 2NO2(g) +
1
2
O2(g) depends
on the concentration of reactant.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-8-2048.jpg)

![Order of Reaction
• For any general reaction occurring at a fixed temperature
aA + bB → cC + dD
• The rate expression has the general form
Rate = k[A]m[B]n
• The power (exponents m and n ) to which the concentration of
reactant is raised in the rate expression is called the order of
reaction.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-11-2048.jpg)

![Order of Reaction
• A reaction has an individual order “with respect to” or “in” each
reactant.
• For the simple reaction A → products:
Rate = k[A]m
• If m is 0, the reaction is said to have zeroth order (Rate independent
of concentration). If m = 1, the reaction is first-order; if m = 2, it is
second-order](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-13-2048.jpg)
![Order of Reaction
• For the reaction aA + bB → cC + dD, Rate = k[A]m[B]n
• If the rate doubles when [A] doubles, the rate depends on [A]1 and
the reaction is first order with respect to A.
• If the rate quadruples when [A] doubles, the rate depends on [A]2
and the reaction is second order with respect to [A].
• If the rate does not change when [A] doubles, the rate does not
depend on [A], and the reaction is zero order with respect to A.
• Reaction order enables us to understand how the reaction depends
on reactant concentrations.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-14-2048.jpg)

![Plots of reactant concentration, [A], vs. time for first-, second-,
and zero-order reactions.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-16-2048.jpg)
![Plots of rate vs. reactant concentration, [A], for first-, second-,
and zero-order reactions.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-17-2048.jpg)
![Individual and Overall Reaction Orders
For the reaction 2NO(g) + 2H2(g) → N2(g) + 2H2O(g):
The rate law is, rate = k[NO]2[H2]
The reaction is second order with respect to NO, first
order with respect to H2 and third order overall.
Note that the reaction is first order with respect to H2
even though the coefficient for H2 in the balanced
equation is 2.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-18-2048.jpg)
![Sample Problem
SOLUTION:
Determining Reaction Orders from Rate
Laws
PLAN: We inspect the exponents in the rate law, not the coefficients
of the balanced equation, to find the individual orders. We add
the individual orders to get the overall reaction order.
(a) The exponent of [NO] is 2 and the exponent of [O2] is 1, so the
reaction is second order with respect to NO, first order
with respect to O2 and third order overall.
PROBLEM: For each of the following reactions, use the given rate law
to determine the reaction order with respect to each
reactant and the overall order.
(a) 2NO(g) + O2(g) → 2NO2(g); rate = k[NO]2[O2]
(b) CH3CHO(g) → CH4(g) + CO(g); rate = k[CH3CHO]3/2
(c) H2O2(aq) + 3I-(aq) + 2H+(aq) →I3
-(aq) + 2H2O(l); rate = k[H2O2][I-]](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-19-2048.jpg)

![Determination of the rate law
• 1. Isolation method
• Isolate each one of the reactants in turn by having all the others in large
excess. i.e if B is in excess and the rate law is = k[A][B]2
• Approximate [B] by [B]o.
• = k[A][B]2 where k = k [B]o.
• Pseudo first order, k = effective rate constant for a given fixed
concentration of B.
• If A is in excess the = k[B]2 with k = k[A]0 pseudosecond order rate
law](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-21-2048.jpg)
![Determining Reaction Orders
• For the general reaction A + 2B → C + D, the rate law will have the form:
Rate = k[A]m[B]n
• To determine the values of m and n, we run a series of experiments in which
one reactant concentration changes while the other is kept constant, and we
measure the effect on the initial rate in each case.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-22-2048.jpg)
![Initial Rates for the Reaction between A and B
Experiment
Initial Rate
(mol/L·s)
Initial [A]
(mol/L)
Initial [B]
(mol/L)
1 1.75x10-3 2.50x10-2 3.00x10-2
2 3.50x10-3 5.00x10-2 3.00x10-2
3 3.50x10-3 2.50x10-2 6.00x10-2
4 7.00x10-3 5.00x10-2 6.00x10-2
[B] is kept constant for experiments 1 and 2, while [A] is doubled.
Then [A] is kept constant while [B] is doubled.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-23-2048.jpg)
![Rate 2
Rate 1
=
k[A] [B]
k[A] [B]
m
2
n
2
m
1
n
1
Finding m, the order with respect to A:
We compare experiments 1 and 2, where [B] is kept
constant but [A] doubles:
=
[A]
[A]
m
2
m
1
=
[A]2
[A]1
m
3.50x10-3 mol/L·s
1.75x10-3mol/L·s
=
5.00x10-2 mol/L
2.50x10-2 mol/L
m
Dividing, we get 2.00 = (2.00)m so m = 1](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-24-2048.jpg)
![Rate 3
Rate 1
=
k[A] [B]
k[A] [B]
m
3
n
3
m
1
n
1
Finding n, the order with respect to B:
We compare experiments 3 and 1, where [A] is kept
constant but [B] doubles:
=
[B]
[B]
n
3
n
1
=
[B]3
[B]1
n
3.50x10-3 mol/L·s
1.75x10-3mol/L·s
=
6.00x10-2 mol/L
3.00x10-2 mol/L
m
Dividing, we get 2.00 = (2.00)n so n = 1](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-25-2048.jpg)
![Initial Rates for the Reaction between O2 and NO
Initial Reactant
Concentrations (mol/L)
Experiment
Initial Rate
(mol/L·s) [O2] [NO]
1 3.21x10-3 1.10x10-2 1.30x10-2
2 6.40x10-3 2.20x10-2 1.30x10-2
3 12.48x10-3 1.10x10-2 2.60x10-2
4 9.60x10-3 3.30x10-2 1.30x10-2
5 28.8x10-3 1.10x10-2 3.90x10-2
O2(g) + 2NO(g) → 2NO2(g) Rate = k[O2]m[NO]n](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-26-2048.jpg)
![Rate 2
Rate 1
=
k[O2] [NO]
k[O2] [NO]
m
2
n
2
m
1
n
1
Finding m, the order with respect to O2:
We compare experiments 1 and 2, where [NO] is kept
constant but [O2] doubles:
=
[O2]
[O2]
m
2
m
1
=
[O2]2
[O2]1
m
6.40x10-3 mol/L·s
3.21x10-3mol/L·s
=
2.20x10-2 mol/L
1.10x10-2 mol/L
m
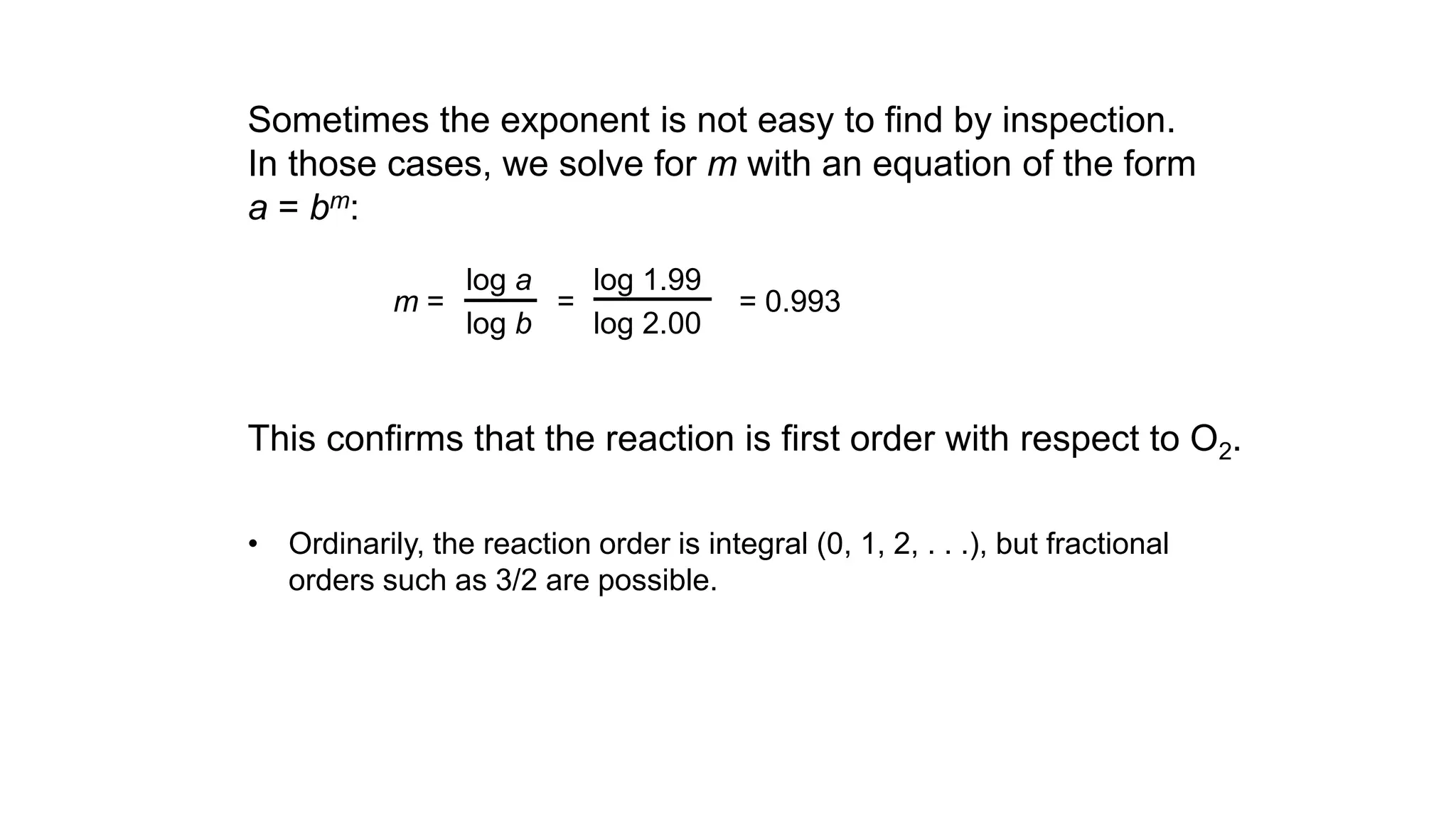
Dividing, we get 1.99 = (2.00)m or 2 = 2m, so m = 1
The reaction is first order with respect to O2.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-27-2048.jpg)
![Finding n, the order with respect to NO:
We compare experiments 1 and 3, where [O2] is kept
constant but [NO] doubles:
Rate 3
Rate 1
=
[NO]3
[NO]1
n
12.8x10-3 mol/L·s
3.21x10-3mol/L·s
=
2.60x10-2 mol/L
1.30x10-2 mol/L
n
Dividing, we get 3.99 = (2.00)n or 4 = 2n, so n = 2.
The reaction is second order with respect to NO.
log a
log b
n =
log 3.99
log 2.00
= = 2.00
Alternatively:
The rate law is given by: rate = k[O2][NO]2](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-29-2048.jpg)
![Determining Reaction Orders from Rate Data
PROBLEM: Many gaseous reactions occur in a car engine and exhaust
system. One of these is
NO2(g) + CO(g) → NO(g) + CO2(g) rate = k[NO2]m[CO]n
Use the following data to determine the individual and
overall reaction orders:
Experiment
Initial Rate
(mol/L·s)
Initial [NO2]
(mol/L)
Initial [CO]
(mol/L)
1 0.0050 0.10 0.10
2 0.080 0.40 0.10
3 0.0050 0.10 0.20](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-30-2048.jpg)
![PLAN: We need to solve the general rate law for m and for n and
then add those orders to get the overall order. We proceed by
taking the ratio of the rate laws for two experiments in which
only the reactant in question changes concentration.
SOLUTION:
rate 2
rate 1
[NO2] 2
[NO2] 1
m
=
k [NO2]m
2[CO]n
2
k [NO2]m
1 [CO]n
1
=
0.080
0.0050
0.40
0.10
=
m
16 = (4.0)m so m = 2
To calculate m, the order with respect to NO2, we compare
experiments 1 and 2:
The reaction is second order in NO2.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-31-2048.jpg)
![k [NO2]m
3[CO]n
3
k [NO2]m
1 [CO]n
1
[CO] 3
[CO] 1
n
=
rate 3
rate 1
=
0.0050
0.0050
=
0.20
0.10
n
1.0 = (2.0)n so n = 0
rate = k[NO2]2[CO]0 or rate = k[NO2]2
To calculate n, the order with respect to CO, we compare experiments
1 and 3:
The reaction is zero order in CO.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-32-2048.jpg)

![SOLUTION:
(a) For reactant A (red):
Experiments 1 and 2 have the same number of particles of B, but
the number of particles of A doubles. The rate doubles. Thus the
order with respect to A is 1.
(b) Rate = k[A][B]2
(c) Between experiments 3 and 4, the number of particles of A
doubles while the number of particles of B does not change. The
rate should double, so rate = 2 x 2.0x10-4 = 4.0x10-4mol/L·s
For reactant B (blue):
Experiments 1 and 3 show that when the number of particles of B
doubles (while A remains constant), the rate quadruples. The
order with respect to B is 2.
The overall order is 1 + 2 = 3.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-34-2048.jpg)

![Information sequence to determine the kinetic parameters of
a reaction.
Series of plots of
concentration vs.
time
Initial rates
Reaction
orders
Rate constant (k)
and actual rate law
Determine slope of tangent at t0 for
each plot.
Compare initial rates when [A] changes and
[B] is held constant (and vice versa).
Substitute initial rates, orders, and
concentrations into rate = k[A]m[B]n, and
solve for k.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-36-2048.jpg)
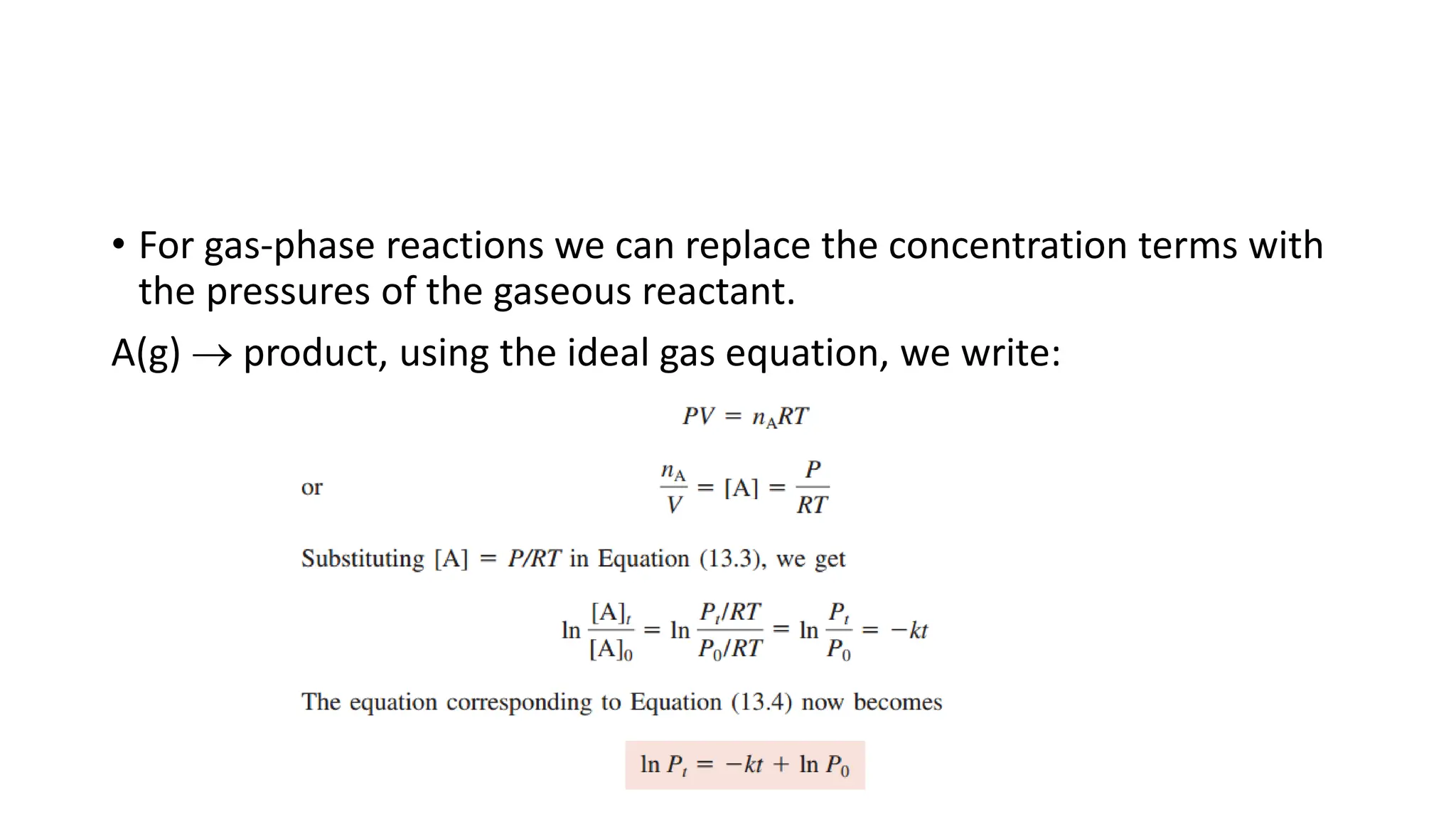
![Reactant Concentration and time
• The rate expression Rate = = k[N2O5], shows how the rate of decomposition of
N2O5 changes with concentration.
• However, it is more important to know the relation between
concentration and time rather than between concentration and rate.
• you would want to know how much N2O5 is left after 5 minutes, 1 hour, or
several days
• The above equation does not provide this information.
• Using calculus, it is possible to develop integrated rate equations relating reactant
concentration to time.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-37-2048.jpg)
![First-Order Reactions
Integrated rate equations for a general
reaction A→ products
rate = −
[𝐴]
𝑡
From the rate law
rate = k[A]
Combining the two equations
rate = −
[𝐴]
𝑡
= k[A]
In differential form the eqn becomes
−
𝑑[𝐴]
𝑑𝑡
= k[A]
Rearranging
−
𝑑[𝐴]
[A]
= 𝑘𝑑𝑡
Integrating btwn 𝑡 = 0 𝑎𝑛𝑑 𝑡 = 𝑡
t = 0 can be any time when we choose
to start monitoring the change in the
concentration of A.
න
[𝐴]0
[𝐴]𝑡
𝑑[𝐴]
[A]
= −𝑘 න
0
𝑡
𝑑𝑡
− ln 𝐴 𝑡 − 𝑙𝑛 𝐴 0 = 𝑘𝑡
ln 𝐴 𝑡 = 𝑙𝑛 𝐴 0 − kt … 1
𝐴 𝑡 = [𝐴]0𝑒−𝑘𝑡 … (2)
(1) is of the form y = mx + b, linear eqn
(2) is used for predicting concentration
of A at any time after the reaction has
started](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-38-2048.jpg)
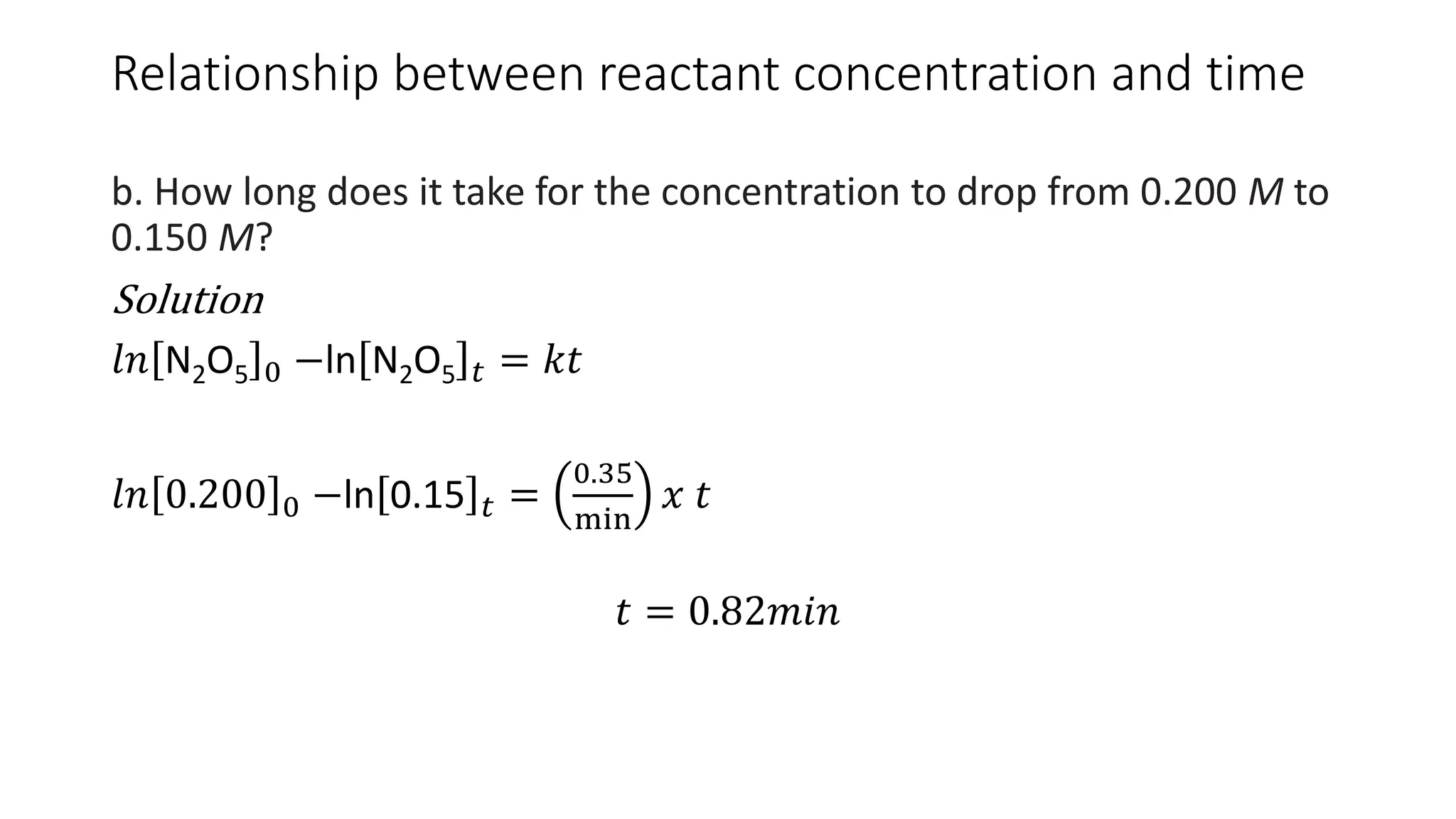
![Relationship between reactant concentration and
time
• As we would expect during the
course of a reaction, the
concentration of the reactant A
decreases with time
• If we plot of In[A]t against t and
get a straight line then the
reaction is first order. Slope is
equal to −𝑘 and a y intercept
equal to 𝑙𝑛 𝐴 0](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-39-2048.jpg)
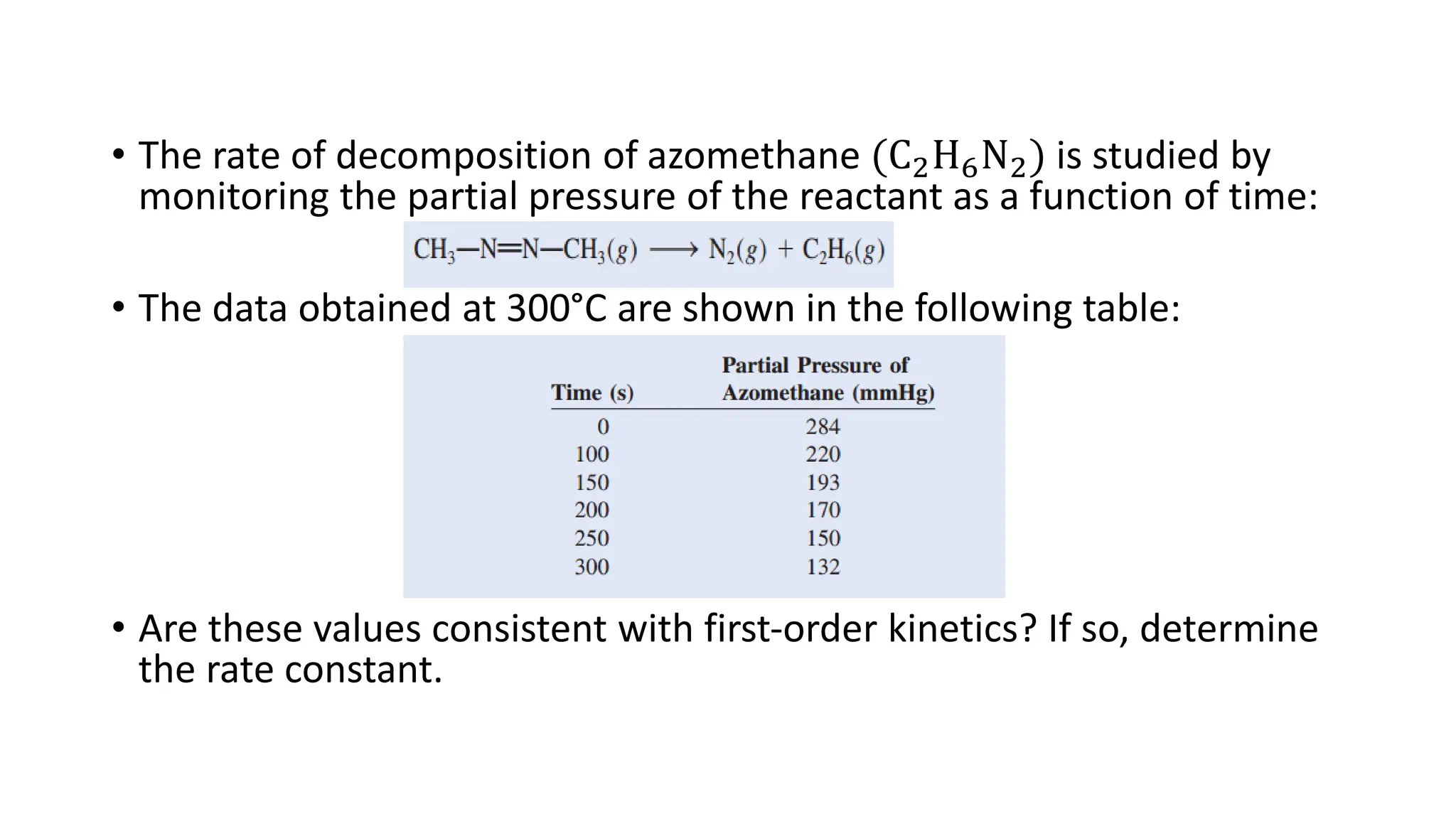
![Exercise: The concentration of N2O5 in liquid bromine varied with
time as follows:
t/s 0 200 400 600 1000
[N2O5]/M 0.110 0.073 0.048 0.032 0.014
Determine the order and rate constant
Order = 1 (first order)
Rate constant = 2.1 x 10-3s-1](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-40-2048.jpg)
![Relationship between reactant concentration and time
Example
Consider the first-order decomposition of N2O5 67°C, where k = 0.35/min.
a. What is the concentration after six minutes, starting at 0.200 M?
Solution
𝑙𝑛 N2O5 0 −ln N2O5 𝑡 = 𝑘𝑡
N2O5 𝑡 = [N2O5]0𝑒−𝑘𝑡
= 0.200𝑥𝑒−(0.35𝑥6)
= 0.024𝑚𝑜𝑙/𝐿](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-41-2048.jpg)

![Graphical method for finding the reaction order from the
integrated rate law.
First-order reaction
ln [A]0
[A]t
= - kt
integrated rate law
ln[A]t = -kt + ln[A]0
straight-line form
A plot of ln [A] vs. time gives a straight line for a first-order reaction.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-47-2048.jpg)
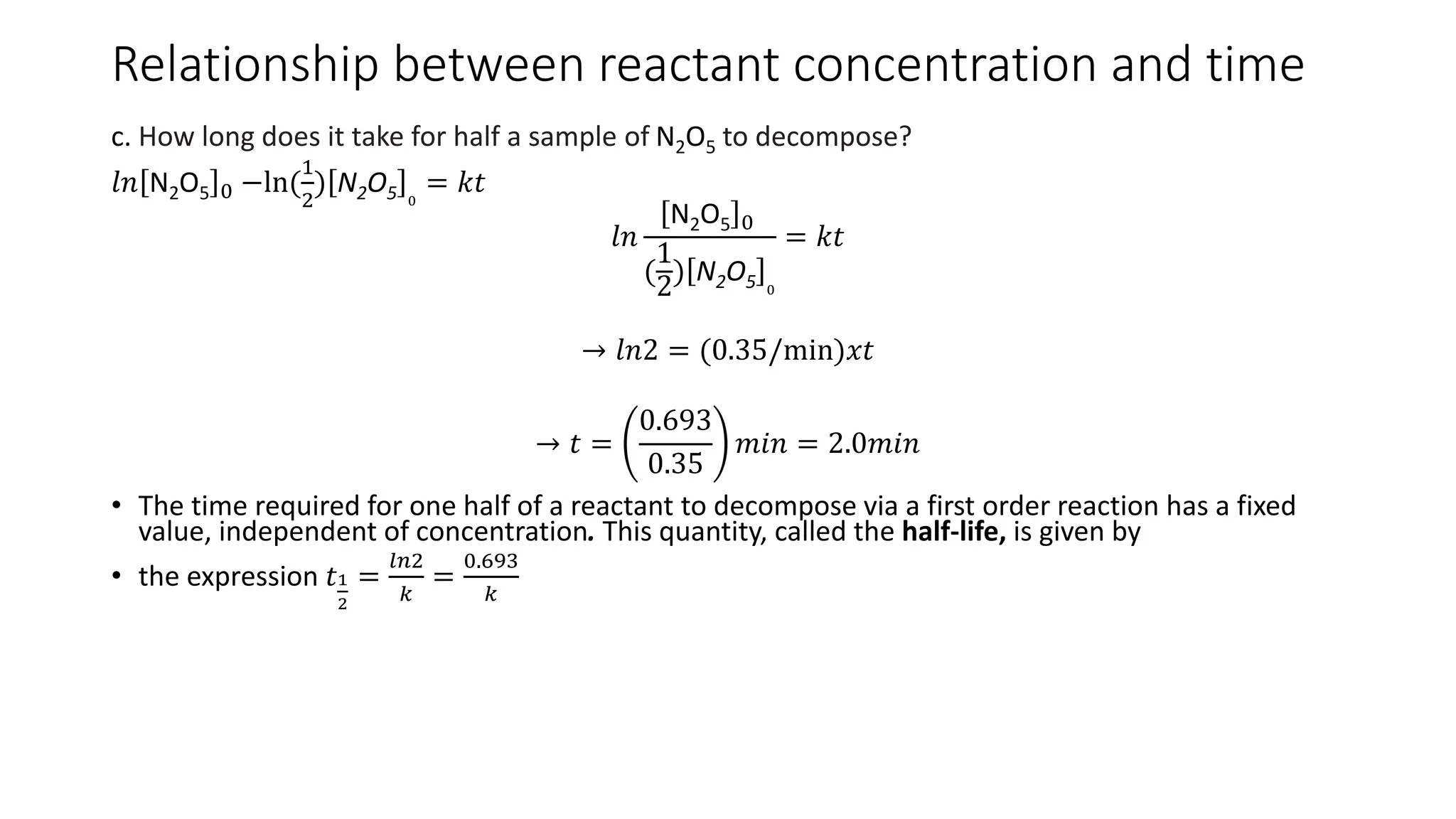
![Half-life method
• Time taken for concentration of reactant to fall to ½ its initial value.
• It’s a guide to the order of a reaction for it depends on initial concentration for
reactions of different orders.
For first order: ln 𝐴 𝑡 = 𝑙𝑛 𝐴 0 − kt
𝑙𝑛 A 0 − ln
1
2
A 0
= 𝑘𝑡1
2
𝑘𝑡 = ln
[𝐴]0
1
2
[𝐴]0
𝑡 1/2 =
𝑙𝑛2
𝑘
=
0.693
𝑘
t1/2: rapid method of measuring 1st order reaction rate
t1/2 for first order reaction is independent of the initial concentration of the
reactant.
will be constant. Not true for second order rxn.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-48-2048.jpg)

![Second-order reaction
• A second-order reaction is a reaction whose rate depends on the concentration
of one reactant raised to the second power or on the concentrations of two
different reactants, each raised to the first power.
• Consider the reaction: A→ products
rate −
𝐴
𝑡
= 𝑘[𝐴]2
• For the reaction A + B→ products:
rate = k A B
• The reaction is first order in A and first order in B, so it has an overall reaction
order of 2](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-50-2048.jpg)
![Second-order reaction
• Integrated rate equations for a general reaction A→ products
𝑟𝑎𝑡𝑒 = −
𝐴
𝑡
න
[𝐴]0
[𝐴]𝑡
𝑑[𝐴]
[A]2
= −𝑘 න
0
𝑡
𝑑𝑡
න 𝑥𝑛
𝑑𝑥 =
𝑥𝑛+1
𝑛 + 1
+ 𝐶
1
𝐴 𝑡
=
1
𝐴 0
+ 𝑘𝑡 … 1
𝐴 𝑡 =
𝐴 0
1 + 𝑘𝑡 𝐴 0
… 2](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-51-2048.jpg)




![Graphical method for finding the reaction order from the
integrated rate law.
Second-order reaction
integrated rate law
1
[A]t
1
[A]0
- = kt
straight-line form
1
[A]t
1
[A]0
= kt +
A plot of vs. time gives a straight line for a second-order reaction.
1
[A]](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-56-2048.jpg)

![Zero-Order Reactions
• First- and second-order reactions are the most common reaction
types. Reactions whose order is zero are rare. For a zero-order
reaction.
A→ products: 𝑟𝑎𝑡𝑒 = 𝑘[𝐴]0 = 𝑘
න
[𝐴]0
[𝐴]𝑡
𝑑[𝐴] = −𝑘 න
0
𝑡
𝑑𝑡
[𝐴]𝑡 = −𝑘𝑡 + [𝐴]0
• A plot of [𝐴]𝑡versus t gives a straight line with slope = -k and y
intercept = [𝐴]0.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-58-2048.jpg)
![Zero-Order Reactions
• The half-life of a zero-order reaction we set [𝐴]𝑡 =
[𝐴]0
2
𝑡1/2 =
𝐴 0
2𝑘
• Most of the zero-order reactions take place at solid surfaces, where
the rate is independent of concentration in the gas phase.
• A typical example is the thermal decomposition of hydrogen iodide on gold:
• When the gold surface is completely covered with HI molecules,
increasing the concentration of HI(g) has no effect on reaction rate.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-59-2048.jpg)
![Graphical method for finding the reaction order from the
integrated rate law.
Zero-order reaction
A plot of [A] vs. time gives a straight line for a first-order reaction.
integrated rate law
[A]t - [A]0 = - kt
straight-line form
[A]t = - kt + [A]0](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-60-2048.jpg)

![1/[HI] vs time gives a linear plot. The reaction
is second-order.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-62-2048.jpg)
![Graphical determination of the reaction order for the decomposition of
N2O5.
The concentration data is used to
construct three different plots. Since the
plot of ln [N2O5] vs. time gives a straight
line, the reaction is first order.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-63-2048.jpg)

![Reactions approaching equilibrium
• At equilibrium, the reverse rate matches the forward rate and the reactants and products are
present in abundances given by the equilibrium constant for the reaction.
• When the reaction has reached equilibrium the concentrations of A and B are [A]eq and [B]eq and
there is no net formation of either substance. It follows that k1[A]eq = k-1[B]eq and therefore that
the equilibrium constant for the reaction is related to the rate constants by
• 𝐾 =
[𝐵]𝑒𝑞
[𝐴]𝑒𝑞
=
𝑘1
𝑘−1
• If the forward rate constant is much larger than the reverse rate constant, then K >> 1. If the
opposite is true, then K << 1. This result is a crucial connection between the kinetics of a reaction
and its equilibrium properties.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-65-2048.jpg)

![Figure 16.14 Increase of the rate constant with temperature for
the hydrolysis of an ester.
Expt [Ester] [H2O] T (K) Rate
(mol/L·s)
k
(L/mol·s)
1 0.100 0.200 288 1.04x10-3 0.0521
2 0.100 0.200 298 2.20x10-3 0101
3 0.100 0.200 308 3.68x10-3 0.184
4 0.100 0.200 318 6.64x10-3 0.332
Reaction rate and k increase exponentially as T increases.](https://image.slidesharecdn.com/chemicalkineticsratelawsandreactionmechanisms-240318015538-a317edc4/75/Chemical-kinetics_Rate-laws-and-reaction-mechanisms-pdf-67-2048.jpg)








