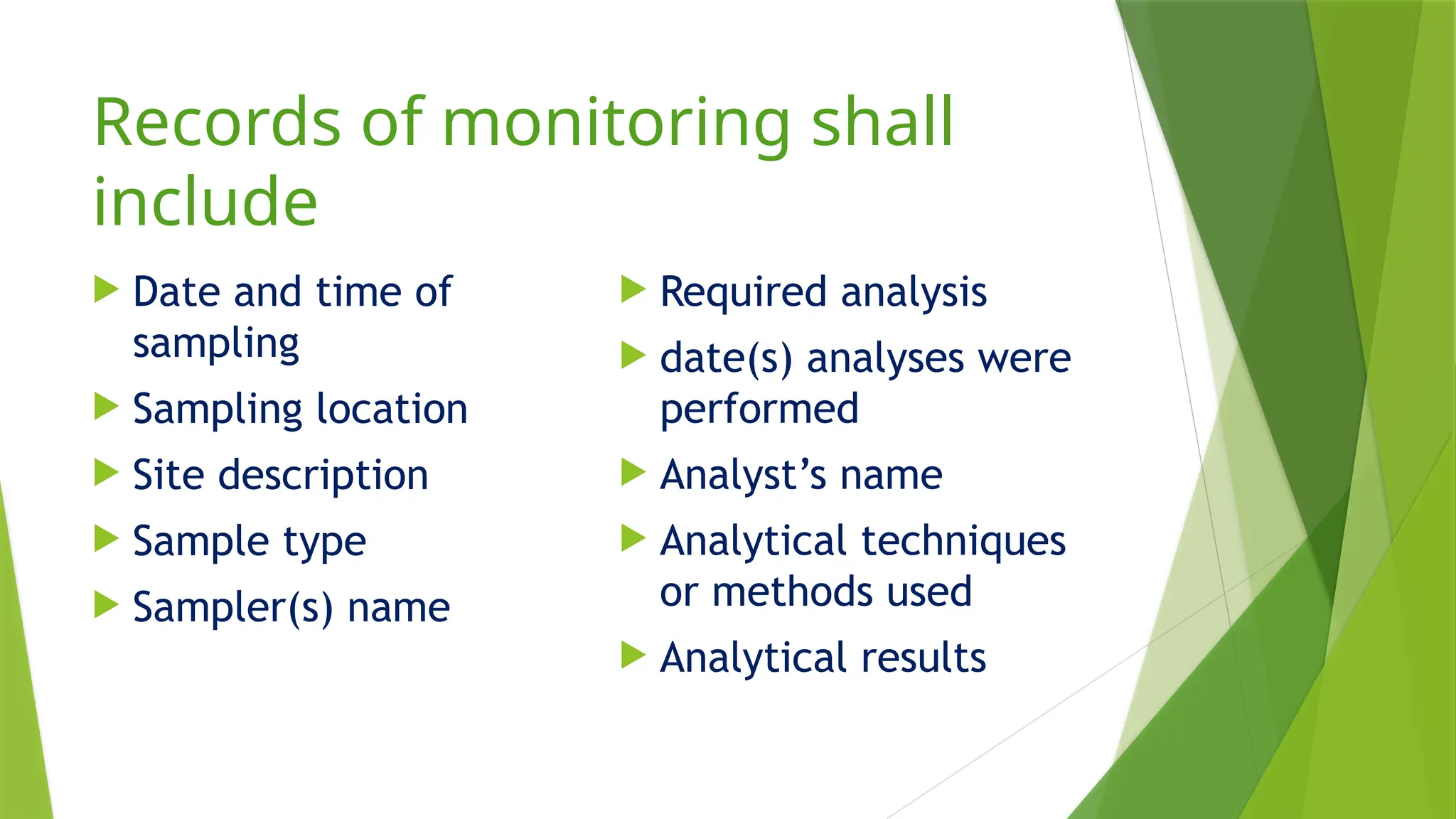
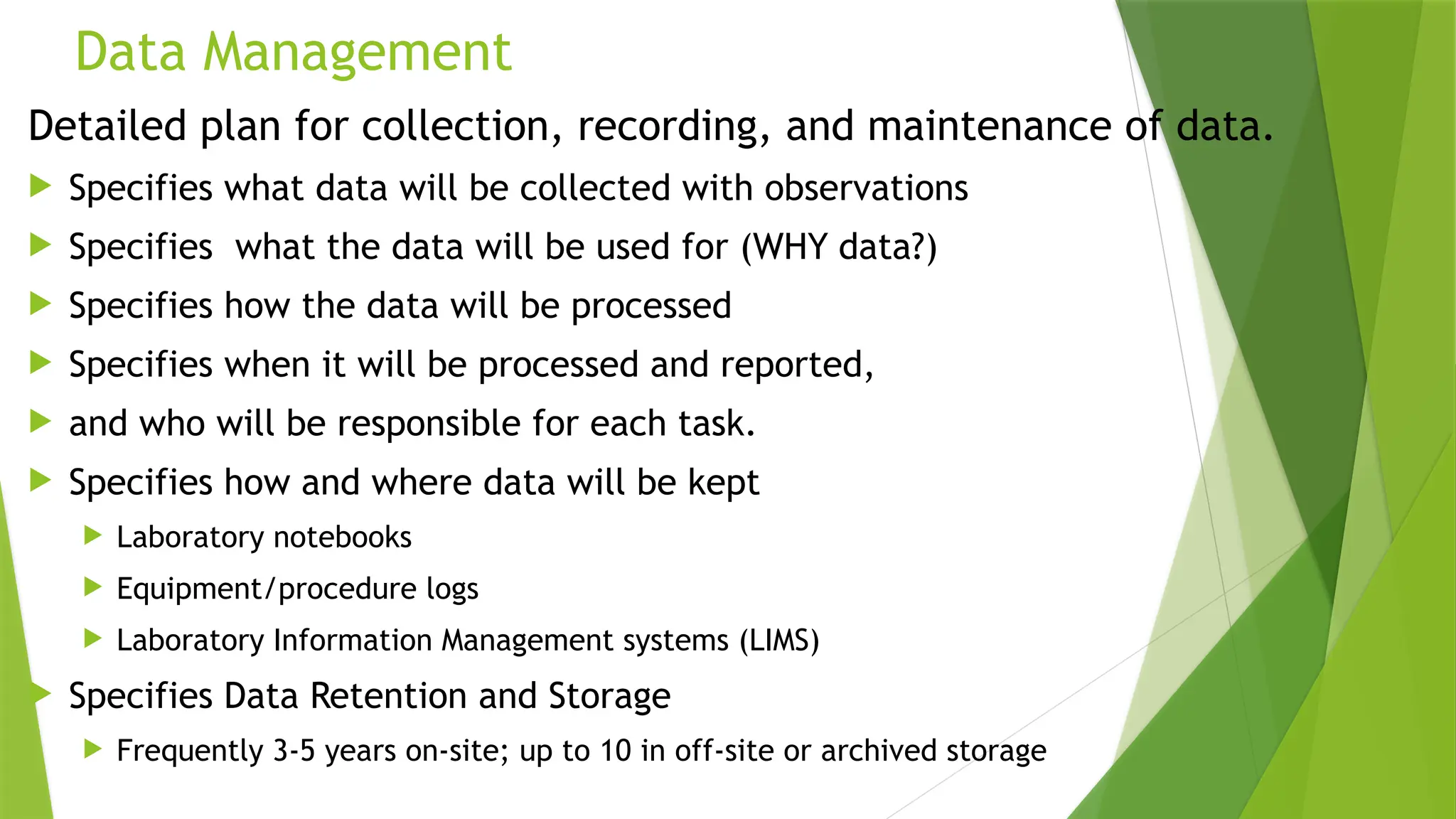
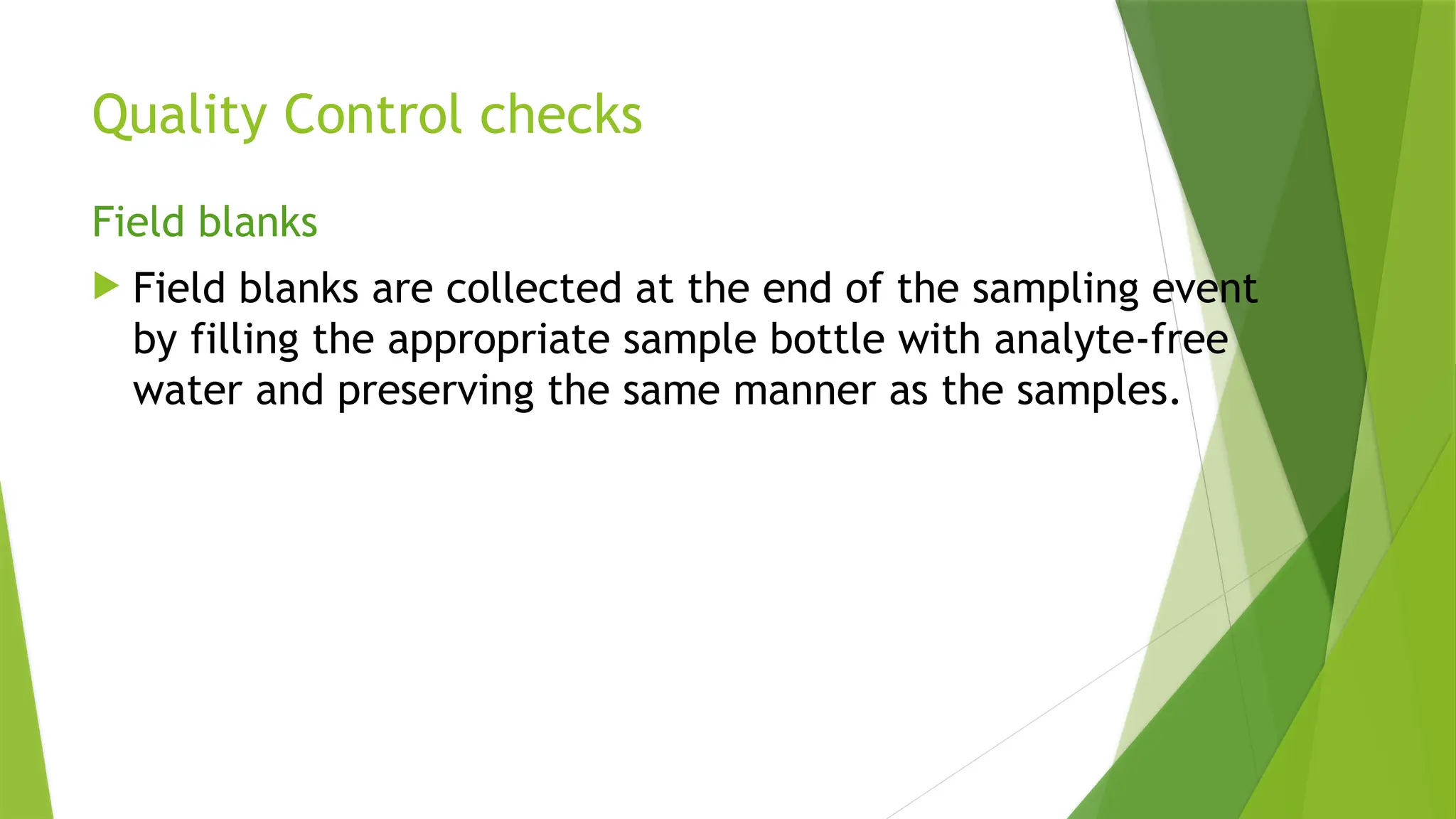
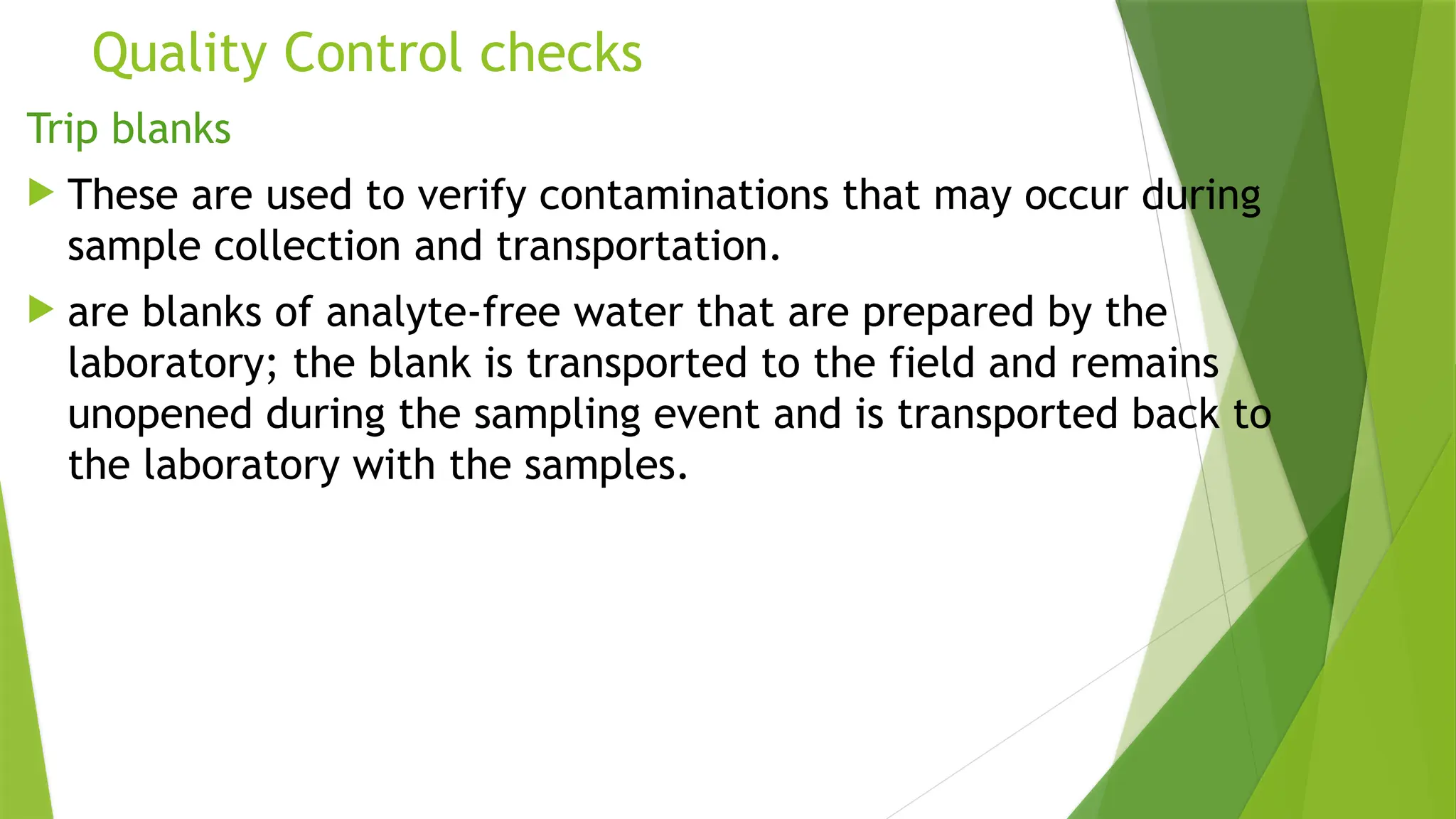
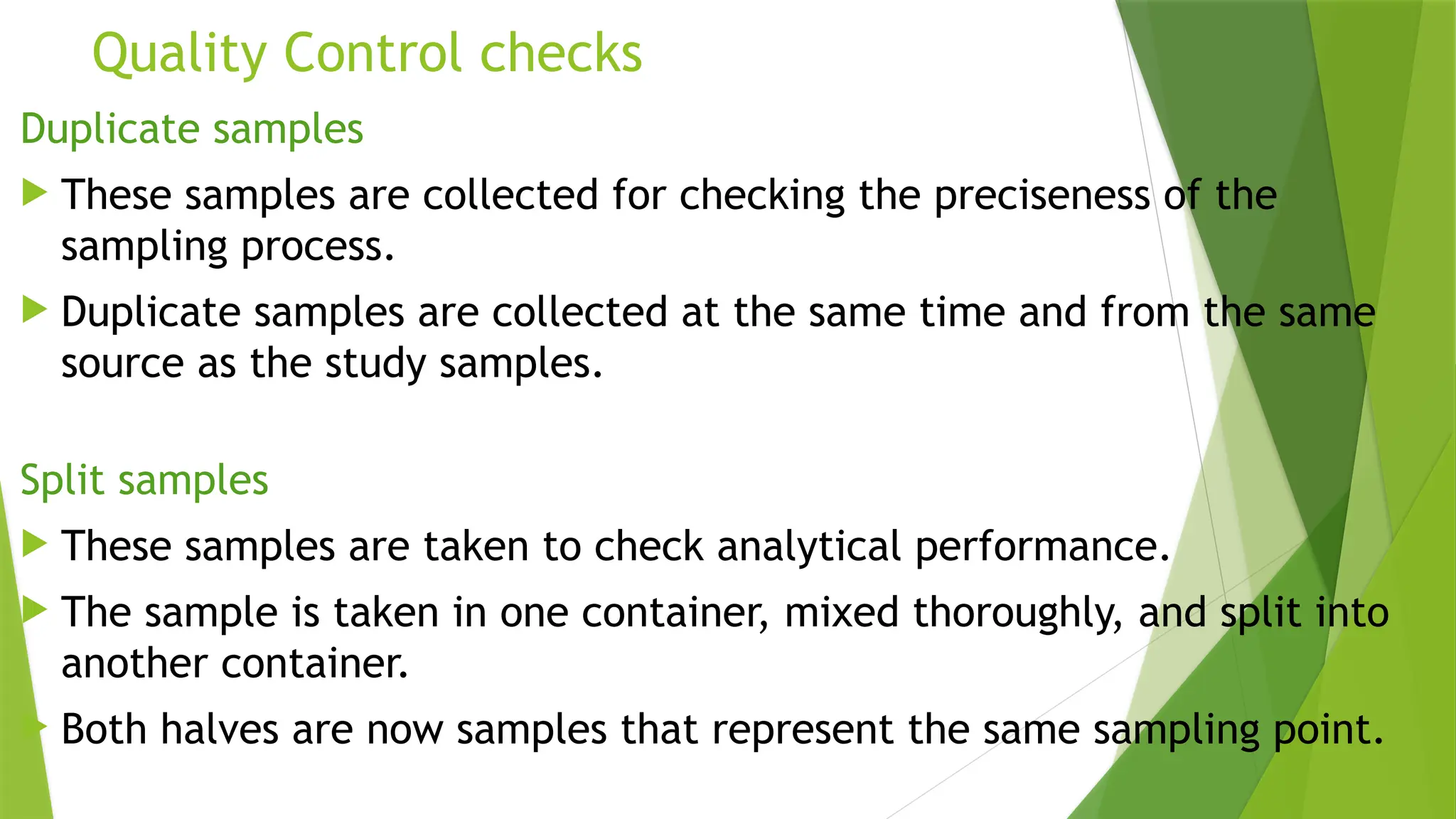
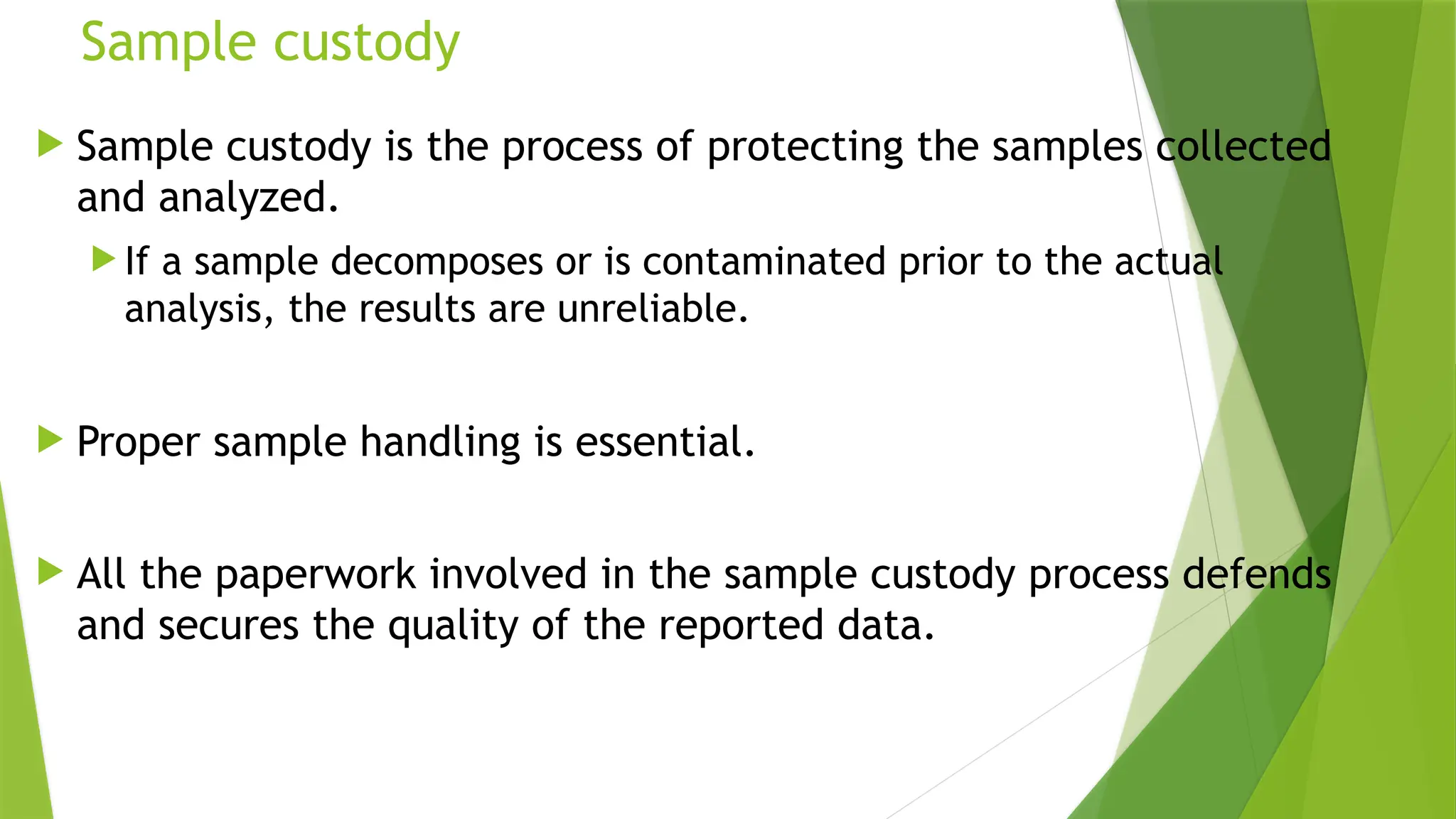
The document outlines the essential components of environmental monitoring data management systems, including detailed guidelines for data collection, storage, and quality assurance procedures. It emphasizes the importance of accurate documentation and the minimization of errors through structured quality control and quality assurance programs. The document also compares different systems such as EMS, EMIS, and EDMS while highlighting the significance of well-designed data management in improving efficiency and minimizing costs in environmental investigations.