The document covers key concepts in chemical kinetics, including the expression of reaction rates, determination of reaction orders, and the methods used to derive rate laws. It outlines various types of rates (initial, instantaneous, and average) and emphasizes the importance of rate laws in understanding reaction mechanisms at a molecular level. Additionally, it details the graphical methods for determining rate laws and explores the half-lives of reactions and the relationship between concentration and reaction rates.
![Instantaneous Rate:
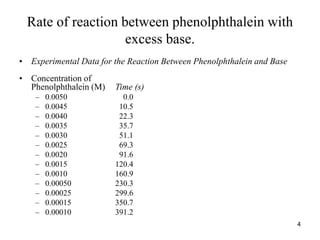
Rate of decrease in [Phenolphthalein]
5](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-5-320.jpg)
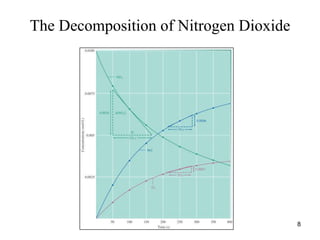
![Average rate during the first 150 s
• Solution:
• Average rate =
• =
• =
• = 3.0 x 10-5
mol/(L.s)
t
]
[NO
- 2
s
150
mol/L)
0.0100
-
mol/L
(0.0055
-
s
150
mol/L
0.0045
10](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-10-320.jpg)
![Average rate during the second 150 s
• Solution:
• Average rate =
• =
• =
• = 1.1 x 10-5
mol/(L.s)
• Average rate decreases as reaction progresses
because the reactant concentration has decreased
t
]
[NO
- 2
s
150
mol/L)
0.0055
-
mol/L
(0.0038
-
s
150
mol/L
0.0017
11](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-11-320.jpg)
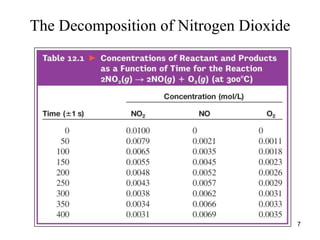
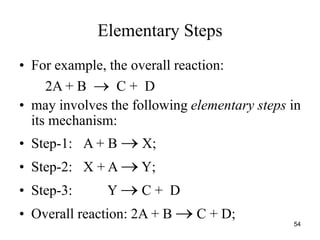
![Rate Law
• Shows how the rate depends on the
concentrations of reactants.
• For the decomposition of nitrogen dioxide:
2NO2(g) → 2NO(g) + O2(g)
Rate = k[NO2]n:
k = rate constant
n = order of the reactant
12](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-12-320.jpg)
![Rate Law
Rate = k[NO2]n
• The concentrations of the products do not
appear in the rate law because the reaction
rate is being studied under conditions where
the reverse reaction does not contribute to the
overall rate.
13](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-13-320.jpg)
![Rate Law
Rate = k[NO2]n
• The value of the exponent n must be
determined by experiment; it cannot be
written from the balanced equation.
14](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-14-320.jpg)
![Rate Law
• An expression or equation that relates the rate
of reaction to the concentrations of reactants at
constant temperature.
• For the reaction:
R1 + R2 + R3 Products
Rate = k[R1]x
[R2]y
[R3]z
Where k = rate constant; x, y, and z are the rate orders
with respect to individual reactants. Rate orders are
determined experimentally.
15](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-15-320.jpg)


![Expressions of Reaction Rates and Their
Stoichiometric Relationships
• Consider the reaction:
2N2O5 4NO2 + O2
• Rate of disappearance of N2O5 =
• Rate of formation of NO2 =
• Rate of formation of O2 =
• Stoichiometric relationships of these rates
•
t
]
NO
[ 2
t
]
[O2
t
]
O
[N 5
2
t
]
[O
)
t
]
NO
[
(
4
1
)
t
]
O
[N
(
2
1 2
2
5
2
20](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-20-320.jpg)

![Rate Laws
• For a general reaction,
aA + bB + eE Products
• The rate law for this reaction takes the form:
• where k is called the "rate constant."
• x, y, and z, are small whole numbers or simple
fractions and they are the rate order with respect to
[A], [B], and [E]. The sum of x + y + z + . . . is called
the “overall order" of the reaction.
22](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-22-320.jpg)
![Types of Rate Laws
• Consider a general reaction:
aA + bB Products
• The rate law is expressed as,
Rate = k[A]x
[B]y
,
Where the exponents x and y are called the rate
order of the reaction w.r.t. the respective reactants;
These exponents are usually small integers or simple
fractions.
23](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-23-320.jpg)
![Types of Rate Laws
1. Zero-Order Reactions
1. In a zero order reaction the rate does not depend
on the concentration of reactant,
2. For example, the decomposition of HI(g) on a
gold catalyst is a zero-order reaction;
3. 2 HI(g) H2(g) + I2(g)
4. Rate = k[HI]0
= k;
(The rate is independent on the concentration of HI)
24](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-24-320.jpg)
![Types of Rate Laws
• First Order Reactions
In a first order reaction the rate is proportional to
the concentration of one of the reactants.
Example, for first-order reaction:
2N2O5(g) 4NO2(g) + O2(g)
• Rate = k[N2O5],
The rate of decomposition of N2O5 is proportional
to [N2O5], the molar concentration of N2O5
25](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-25-320.jpg)
![Types of Rate Laws
• Second Order Reactions
In a second order reaction, the rate is proportional
to the second power of the concentration of one of
the reactants.
Example, for the decomposition of NO2 follows
second order w.r.t. [NO2]
2NO2(g) 2NO(g) + O2(g)
• Rate = k[NO2]2
26](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-26-320.jpg)
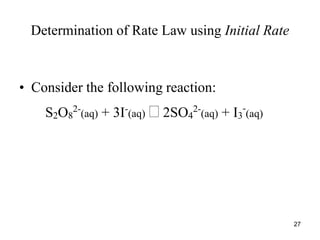
![Determination of Rate Law using Initial Rate
Reaction: S2O8
2-
(aq) + 3I-
(aq) 2SO4
2-
(aq) + I3
-
(aq)
• The following data were obtain.
•
• Expt. [S2O8
2-
] [I-
] Initial Rate,
• # (mol/L) (mol/L) (mol/L.s)
•
• 1 0.036 0.060 1.5 x 10-5
• 2 0.072 0.060 2.9 x 10-5
• 3 0.036 0.120 2.9 x 10-5
•
28](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-28-320.jpg)
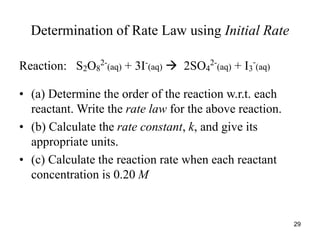
![Determination of Rate Law using Initial Rate
• Solution: The rate law = Rate = k[S2O8
2-
]x
[I-
]y
,
here x and y are rate orders.
• (a) Calculation of rate order, x:
•
1
2
2
~
mol/L.s
10
x
1.5
mol/L.s
10
x
2.9
2
)
060
.
0
(
)
036
.
0
(
)
060
.
0
(
)
072
.
0
(
]
[I
]
O
[S
]
I
[
]
O
[S
5
-
5
-
1
-
1
-
2
8
2
3
-
2
-
2
8
2
y
M
M
k
M
M
k
k
k
x
x
y
x
y
x
y
x
y
x
30](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-30-320.jpg)
![Determination of Rate Law using Initial Rate
• (b) Calculation of rate order, y:
•
• This reaction is first order w.r.t. [S2O8
2-
] and [I-
]
• Rate = k[S2O8
2-
][I-
]
1
2
2
~
mol/L.s
10
x
1.5
mol/L.s
10
x
2.9
2
)
060
.
0
(
)
036
.
0
(
)
120
.
0
(
)
036
.
0
(
]
[I
]
O
[S
]
I
[
]
O
[S
5
-
5
-
1
-
-
2
8
2
3
-
-
2
8
2
y
M
M
k
M
M
k
k
k
y
y
y
x
y
x
y
x
y
x
31](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-31-320.jpg)
![Calculating rate constant and rate at different
concentrations of reactants
• Rate constant, k =
• = 6.6 x 10-3
L.mol-1
.s-1
• If [S2O8
2-
] = 0.20 M, [I-
] = 0.20 M, and
– k = 6.6 x 10-3
L.mol-1
.s-1
• Rate = (6.6 x 10-3
L.mol-1
.s-1
)(0.20 mol/L)2
• = 2.6 x 10-4
mol/(L.s)
mol/L)
60
mol/L)(0.0
(0.038
mol/L
10
x
1.5 -5
32](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-32-320.jpg)
![Integrated Rate Law
• Graphical method to derive the rate law
of a reaction:
• Consider a reaction with single reactant:
• R Products
• If the reaction is zero-order w.r.t. [R],
• Then,
k
Rate
t
[R]
-
33](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-33-320.jpg)
![Graphical Method for Zero-Order Reaction
k
Rate
t
[R]
-
[R] = -kt, and [R]t = [R]0 = kt;
A plot of [R]t versus t yields a straight
line with k = -slope.
34](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-34-320.jpg)
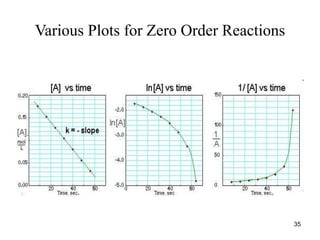
![Graph of Zero-order Reactions
• Plot of [R]t versus t:
[R]t
t
slope = -k
36](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-36-320.jpg)
![Graphical Method for First Order Reactions
• If the reaction: R Products is a first order
reaction, then
• Which yields:
• And a plot of ln[R]t versus t will yield a straight line
with slope = -k and y-intercept = ln[R]0
[R]
t
[R]
-
k
Rate
t
-
ln[R]
ln[R]
t;
-
[R]
[R]
0
t k
k
37](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-37-320.jpg)
![Graph of First Order Reactions
Plot of ln]R]t versus t:
ln[R]t
t
slope = -k
38](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-38-320.jpg)
![Plots of [A] and ln[A] versus time for First Order
Reactions
39](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-39-320.jpg)
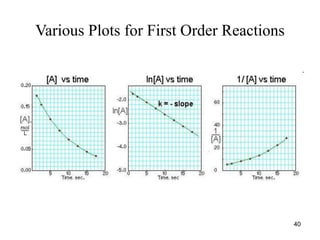
![Graphical Method for Second Order Reactions
• If the reaction: R Products follows second-
order kinetics, then
• or
•
• and
• A plot of 1/[R]t versus t will yield a straight line
with slope = k and y-intercept = 1/[R]0
2
[R]
t
[R]
-
k
Rate
t
[R]
[R]
2
k
[R]
1
t
[R]
1
0
t
k
41](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-41-320.jpg)
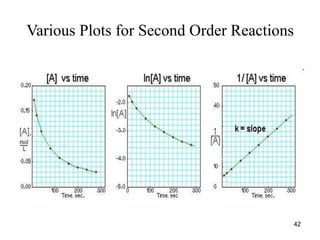
![Graph of Second-order Reactions
• Plot of 1/[R]t versus time:
t
R]
[
1
time
slope = k
43](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-43-320.jpg)
![Plots of ln[Concentration] versus time
45](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-45-320.jpg)
![Plots of 1/[Concn.] versus time
46](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-46-320.jpg)
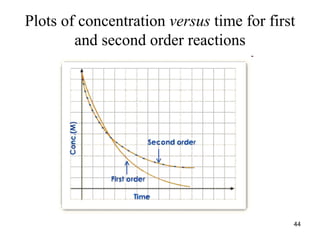
![Characteristics of plots for zero, first, and second
order reactions
• The graph that is linear indicates the order of the
reaction with respect to A (reactant):
• For a zero order reaction, Rate = k (k = - slope)
• For a 1st order reaction, Rate = k[A] (k = - slope)
• For a 2nd order reaction, Rate = k[A]2
(k = slope)
• For zero-order reaction, half-life, t1/2 = [R]0/2k;
• For first order reaction, half-life, t1/2 = 0.693/k;
• For second order reaction, half-life, t1/2 = 1/k[R]0;
47](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-47-320.jpg)
![Half-Lives of Reactions
• For zero-order reaction: t1/2 = [R]0/2k;
• For first-order reaction: t1/2 = 0.693/k;
• For second-order reaction: t1/2 = 1/(k[R]0)
• Note: For first-order reaction, the half-life is
independent of the concentration of reactant, but for
zero-order and second-order reactions, the half-lives
are dependent on the initial concentrations of the
reactants.
48](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-48-320.jpg)


![Exercise
Consider the reaction aA Products.
[A]0 = 5.0 M and k = 1.0 x 10–2 (assume the
units are appropriate for each case). Calculate
[A] after 30.0 seconds have passed, assuming
the reaction is:
a) Zero order
b) First order
c) Second order
4.7 M
3.7 M
2.0 M
52](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-52-320.jpg)
![Molecularity in Elementary Steps
• Molecularity in the number of molecular species that react in
an elementary process.
• Rate Law for Elementary Processes:
• Elementary Reactions Molecularity Rate Law
•
• A product Unimolecular Rate = k[A]
• 2A product Bimolecular Rate = k[A]2
• A + B product Bimolecular Rate = k[A][B]
• 2A + B product Termolecular Rate = k[A]2
[B]
•
55](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-55-320.jpg)
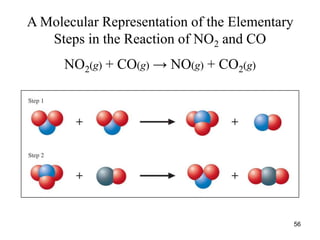
![Reaction Mechanism
• Step-1: NO2 + NO2 NO3 + NO
• Step-2: NO3 + CO NO2 + CO2
• Overall: NO2 + CO NO + CO2
• The experimental rate law is Rate = k[NO2]2
• Which implies that the above reaction is second-order
w.r.t. NO2 , but is zero-order in [CO].
57](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-57-320.jpg)



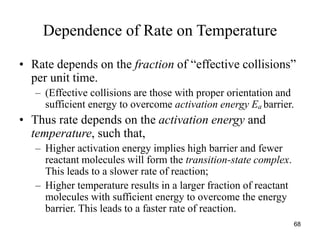
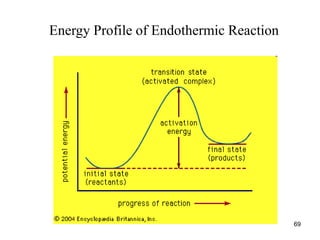
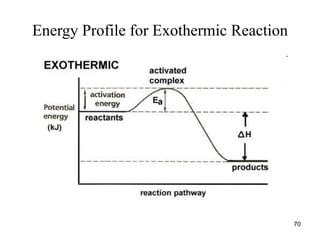
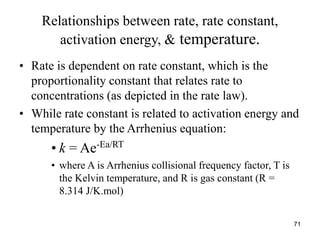
![Dependence of Rate on Concentration
• This is contained in the rate law – that is, for
reaction:
aA + bB + cC Products
Rate = k[A]x
[B]y
[C]z
;
67](https://image.slidesharecdn.com/chemicalkinetics-1a-240613235737-4e3353ea/85/Chemical-kinetics-and-reaction-lecture-notes-67-320.jpg)