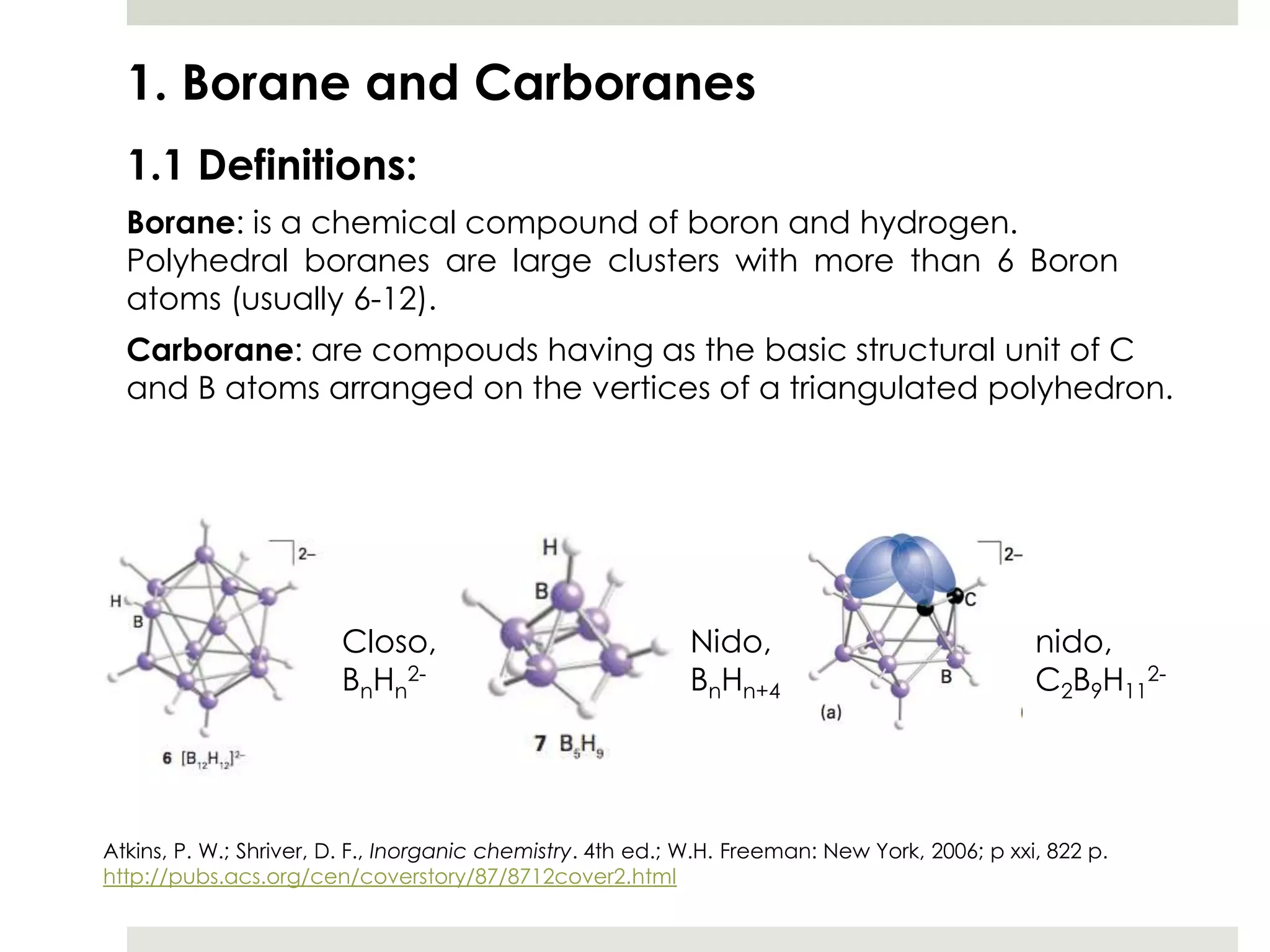
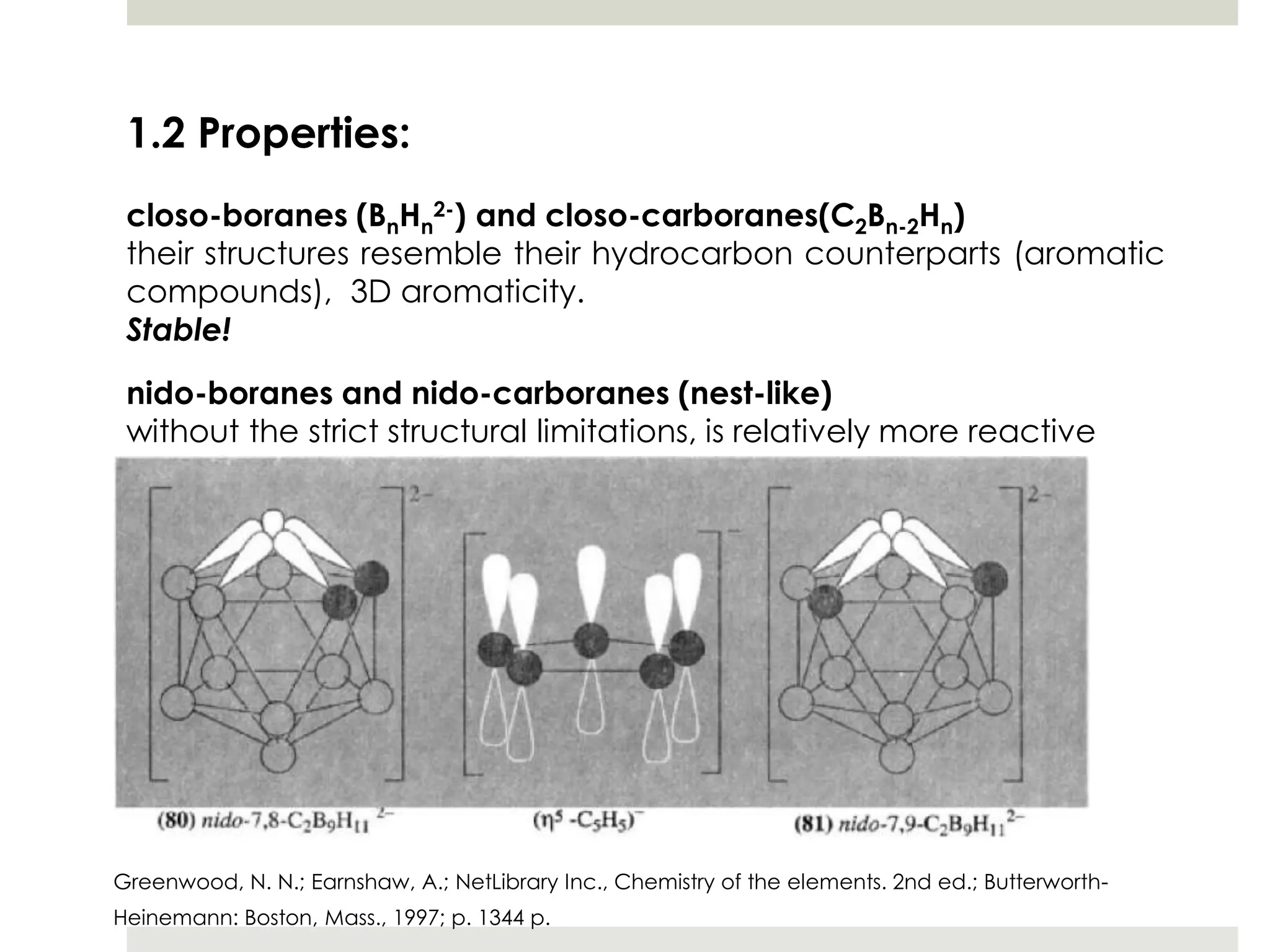
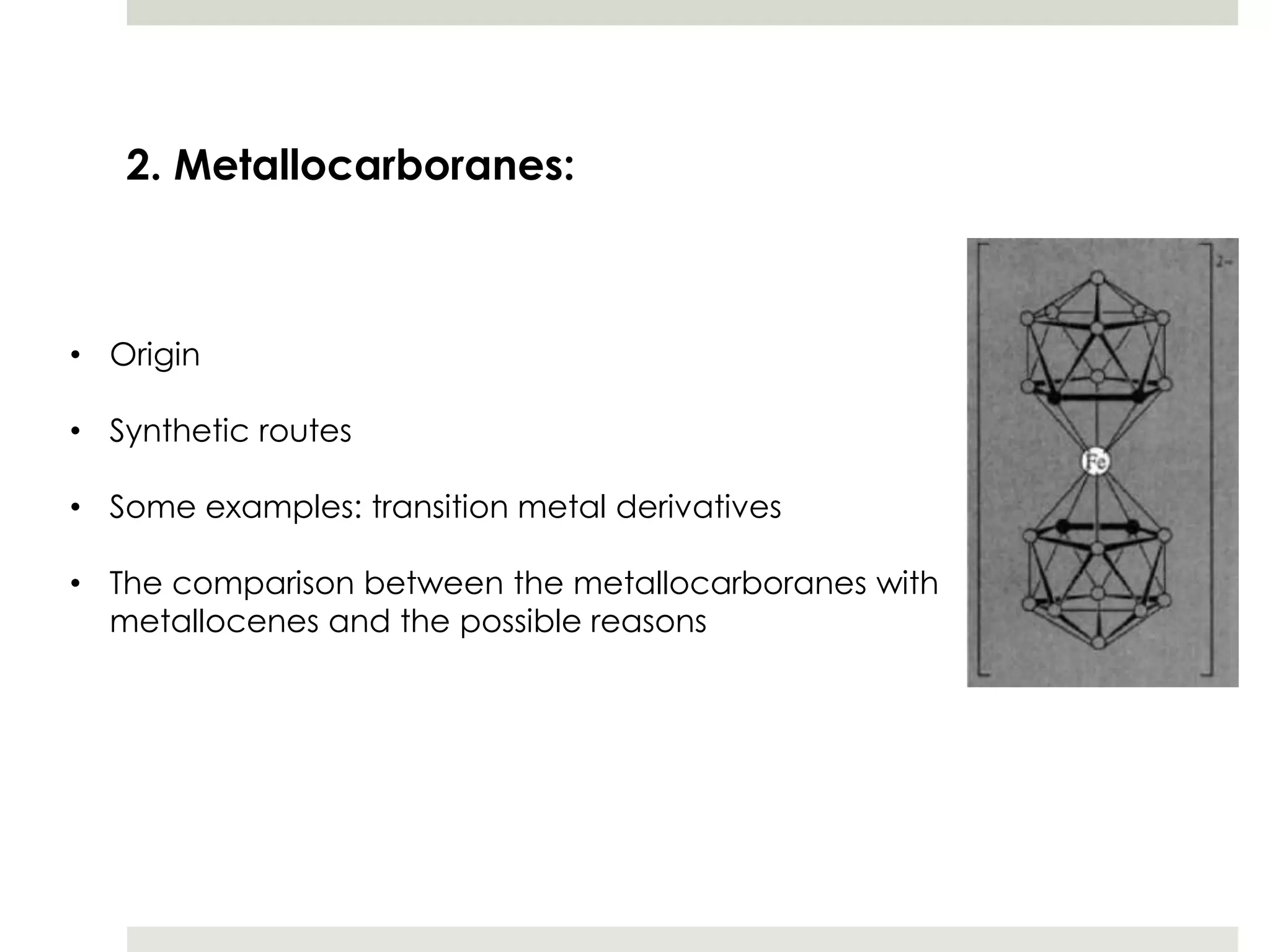
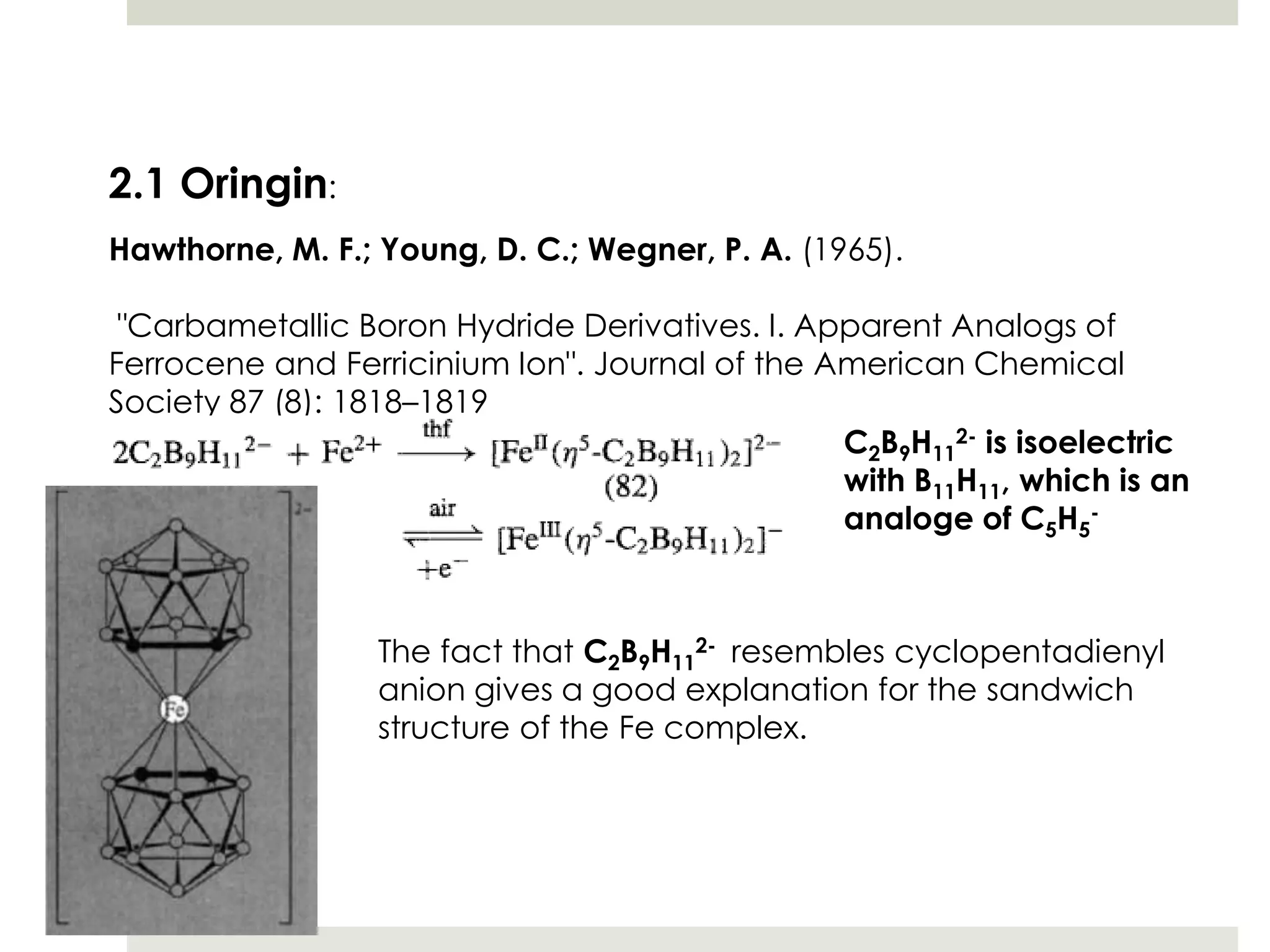
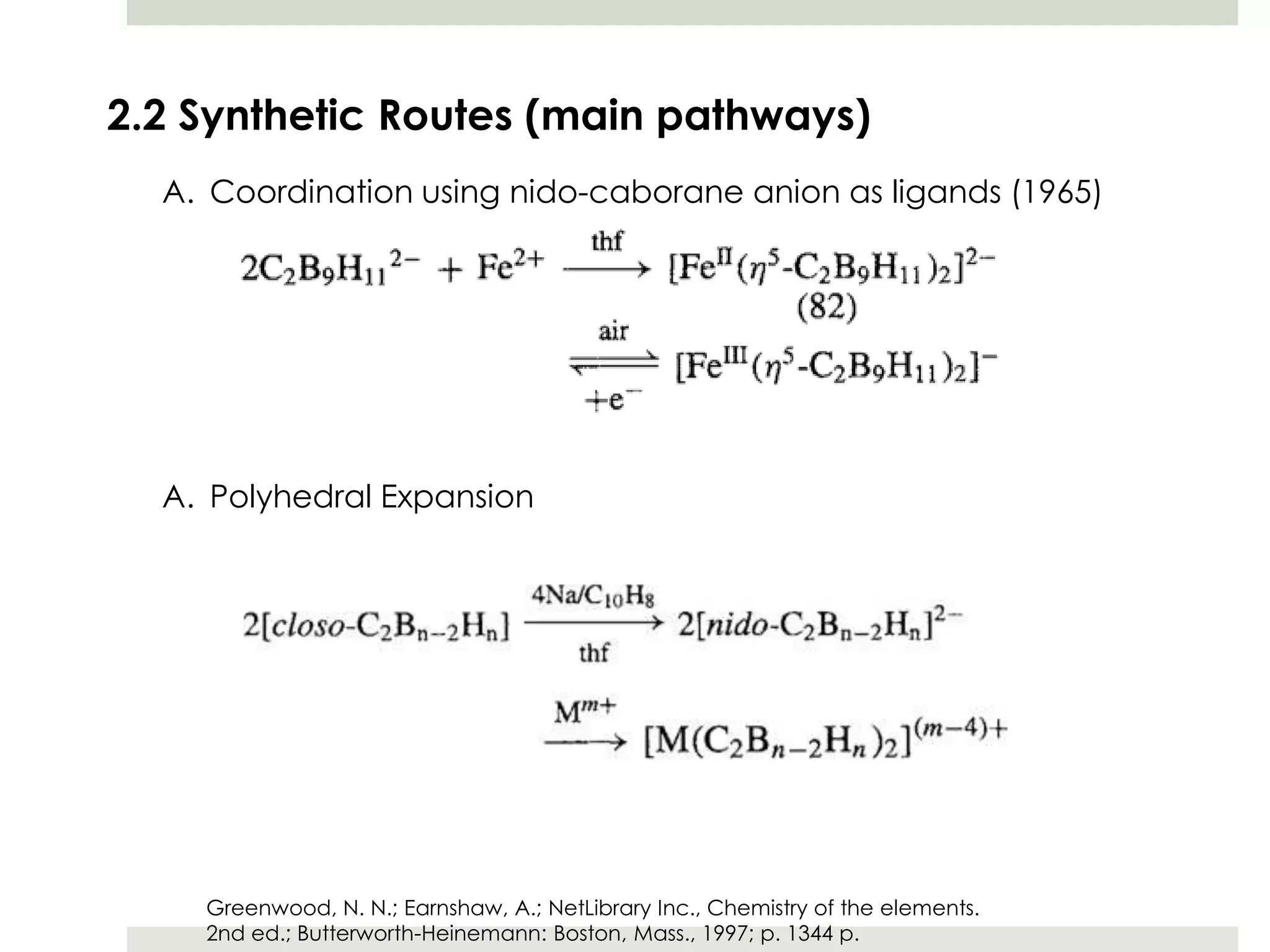
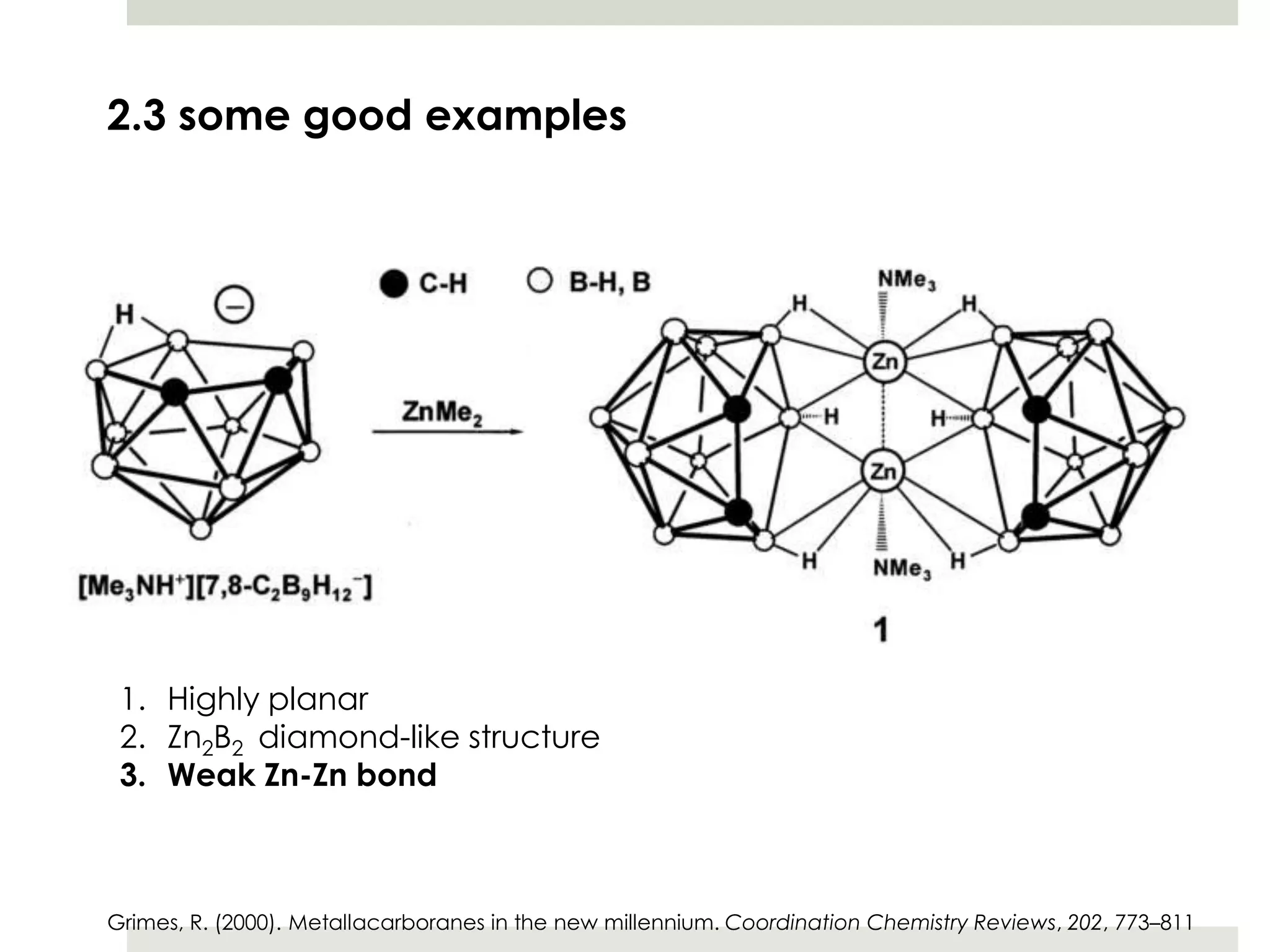
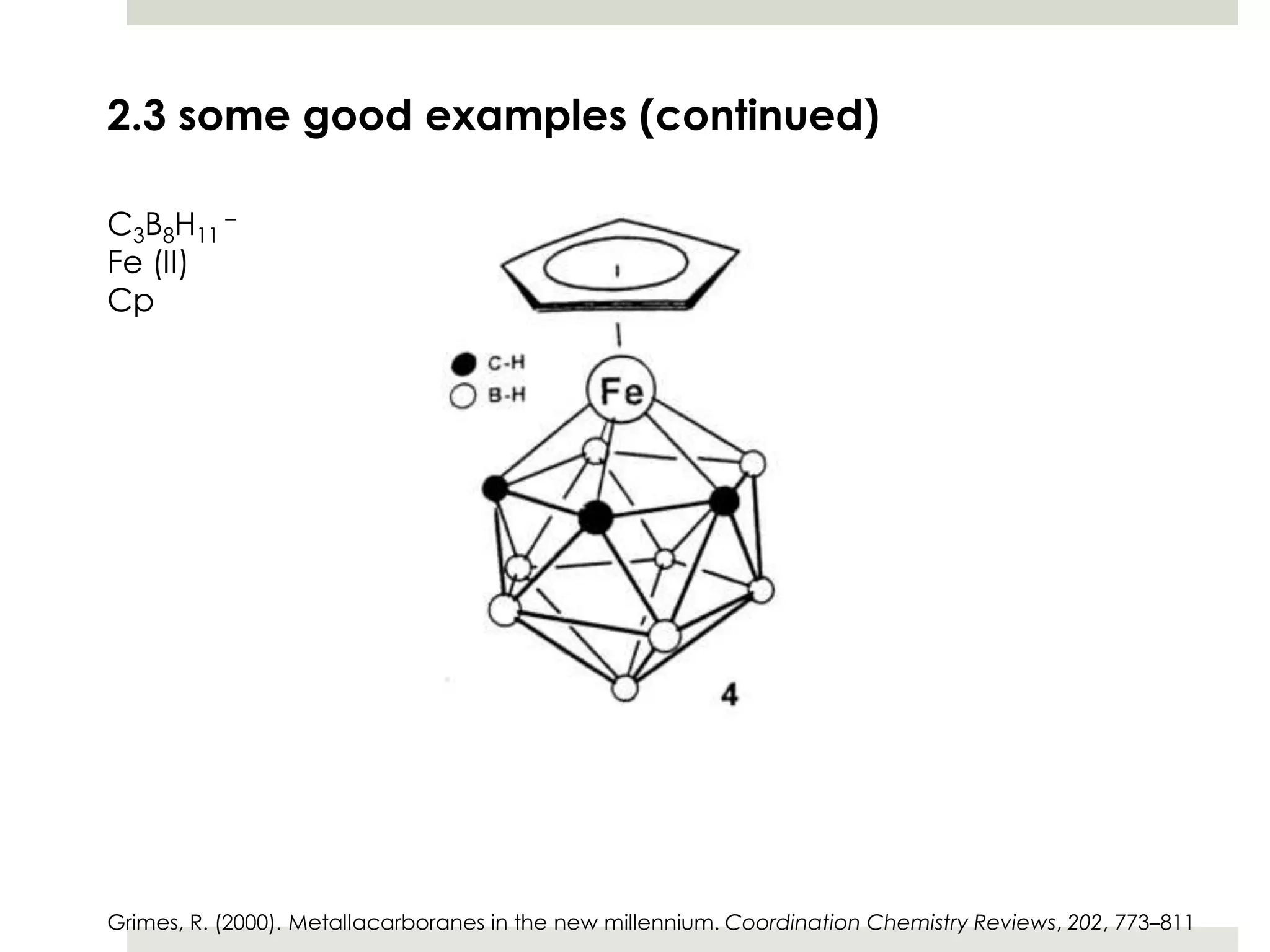
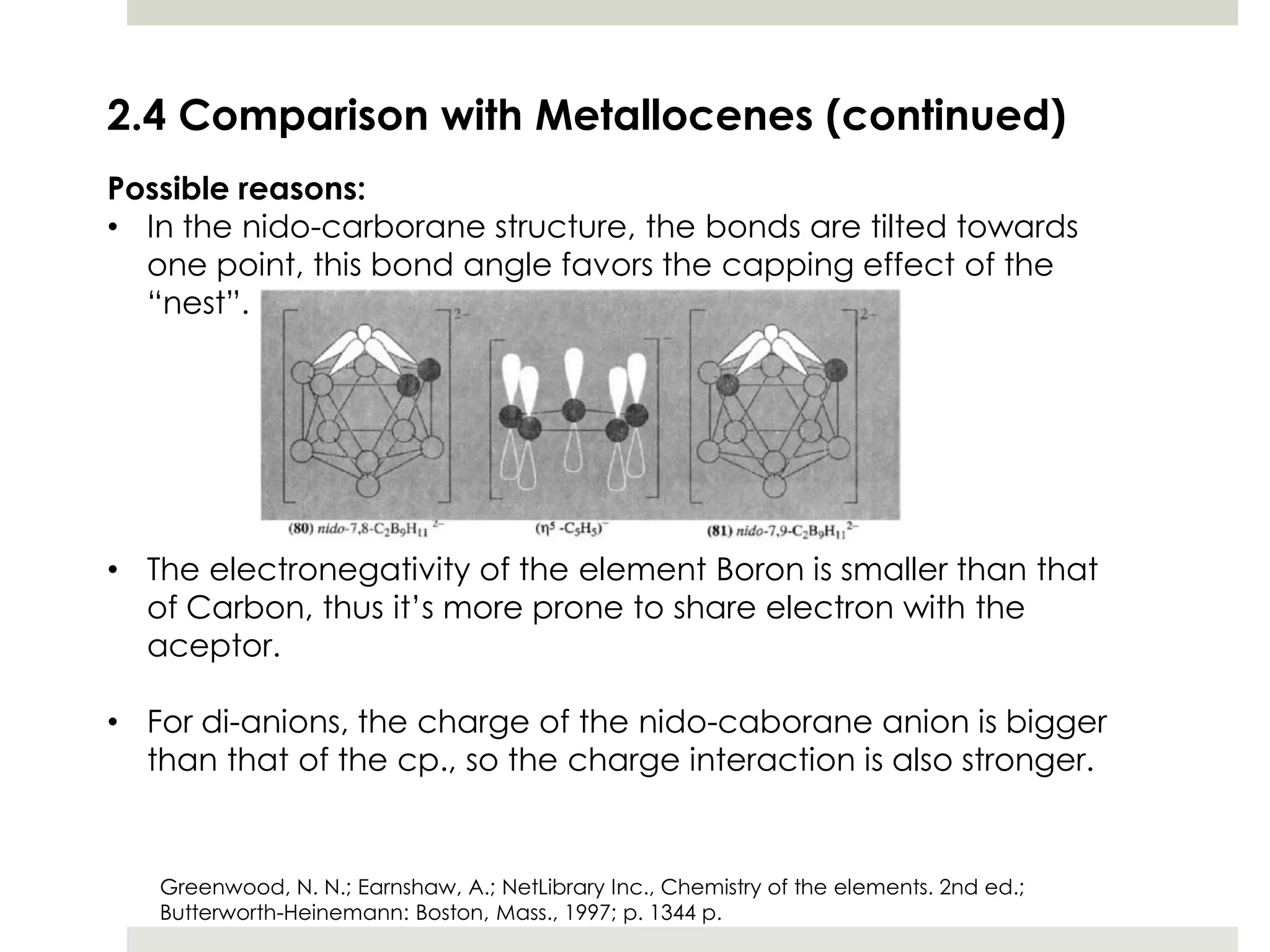
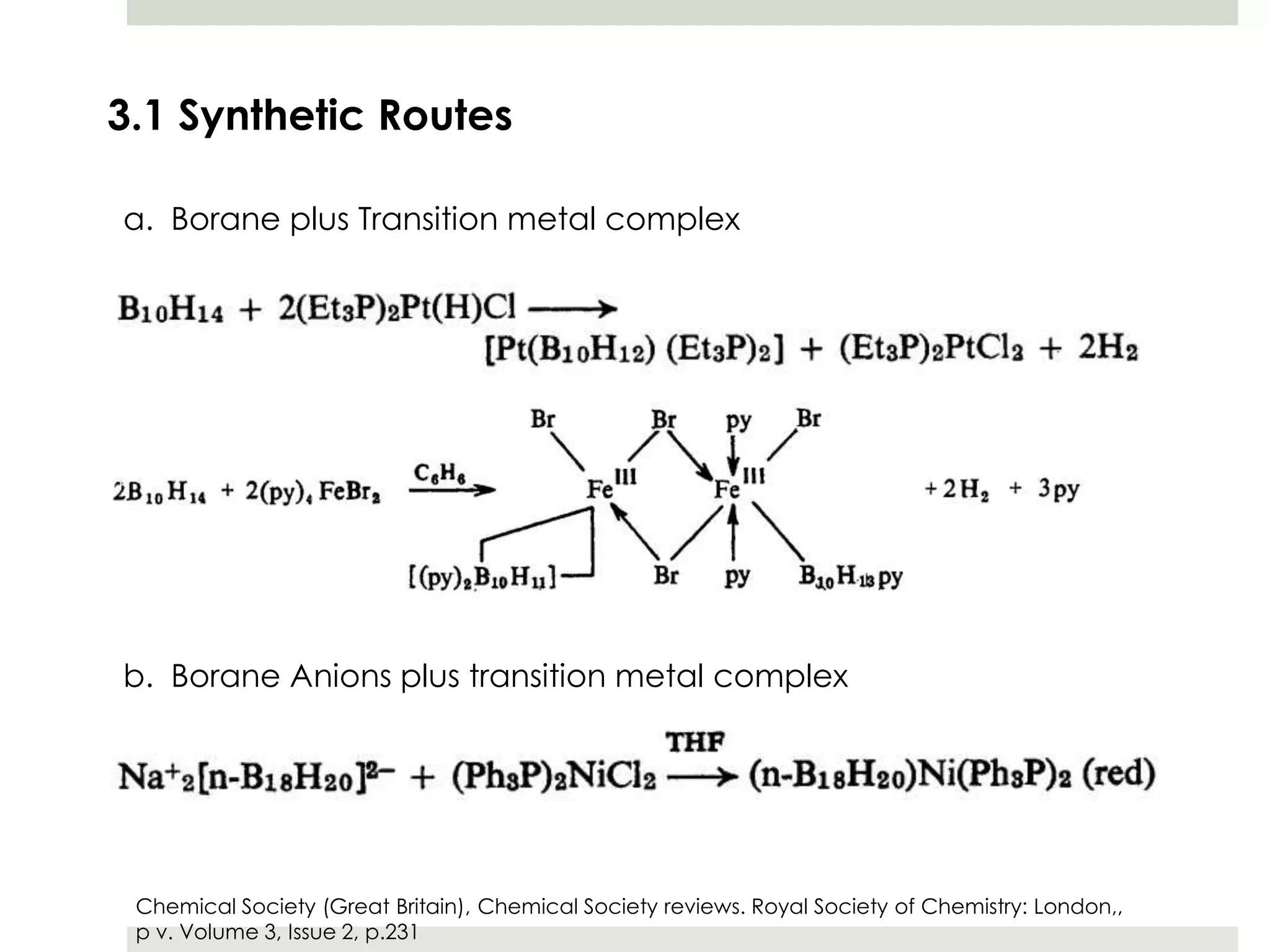
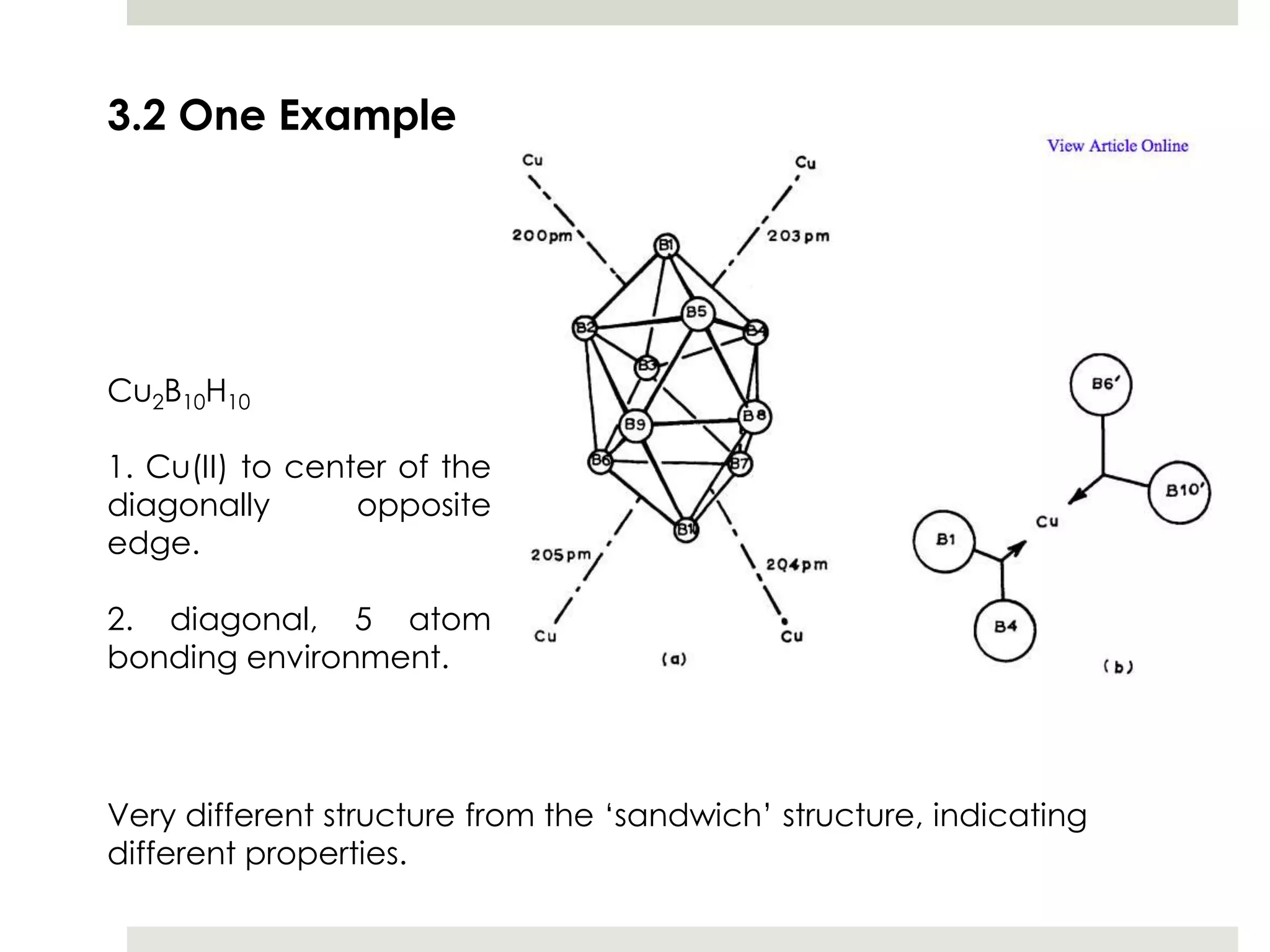
Transition metal derivatives of polyhedral boranes and carboranes can form in different ways. Metallocarboranes often form "sandwich" structures where the metal is bonded between two closo-carborane ligands. These structures are more stable than metallocenes due to properties of the carborane ligands. Metal derivatives of polyhedral boranes can form direct bonds to boron atoms or ionic bonds to the cluster. One example is Cu2B10H10, which has a unique diagonal bonding structure unlike the typical "sandwich". These compounds have various applications including catalysis, organic synthesis, and medicine.