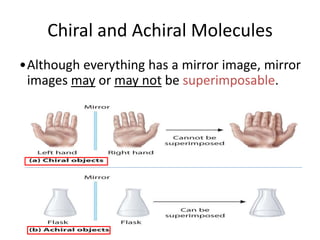
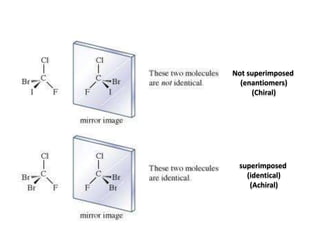
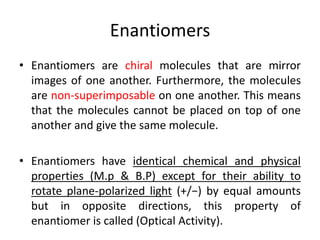
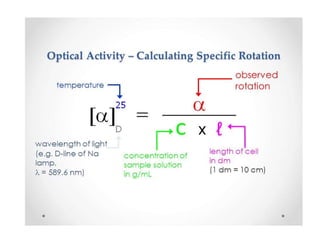
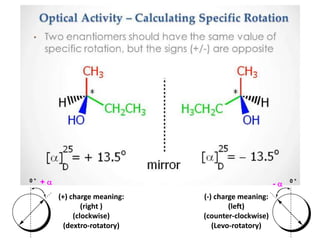
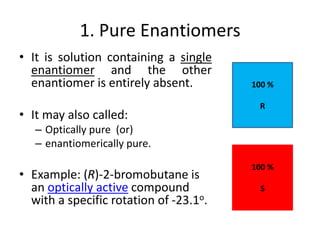
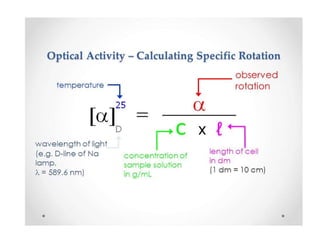
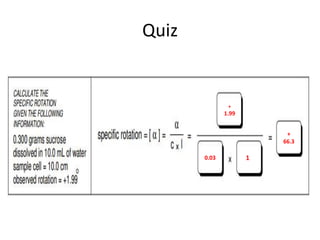
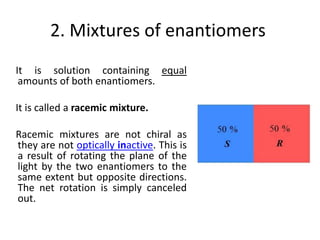
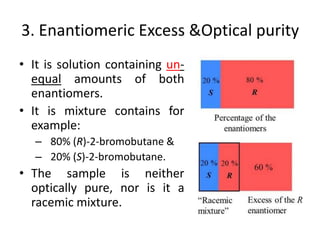
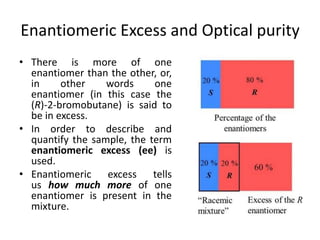
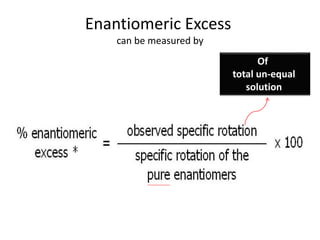
This document discusses chiral and achiral molecules, specifically enantiomers. It defines enantiomers as chiral molecules that are non-superimposable mirror images of each other. While enantiomers have identical chemical and physical properties, they differ in their ability to rotate plane-polarized light in opposite directions by equal amounts. This optical activity property is known as specific rotation. Racemic mixtures contain equal amounts of both enantiomers and are optically inactive, while enantiomeric excess describes mixtures with unequal amounts, neither being pure nor racemic. The document provides examples of calculating enantiomeric excess and optical purity percentages in chiral mixtures.