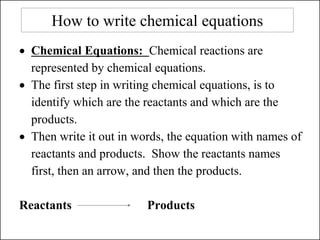
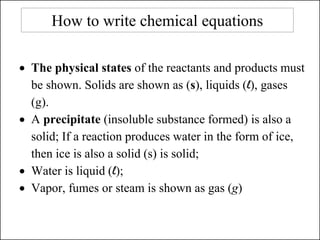
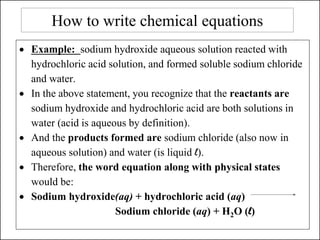
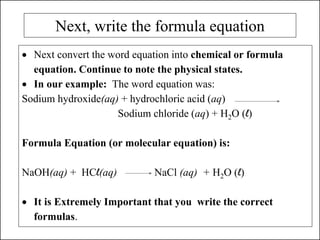
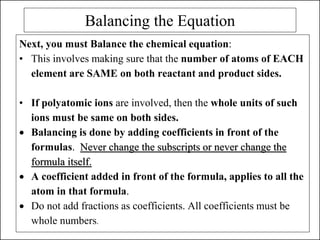
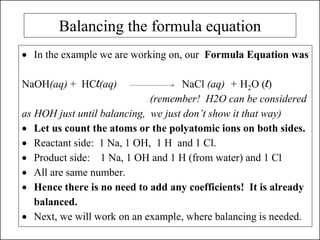
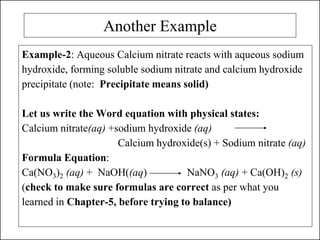
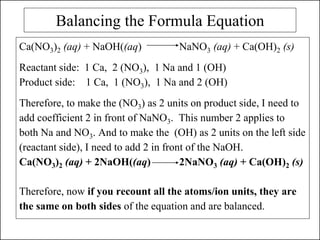
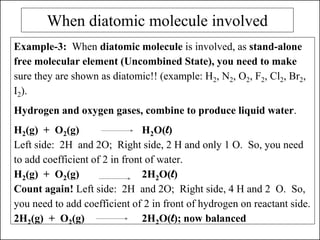
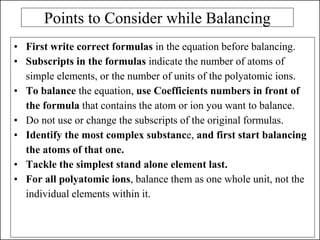
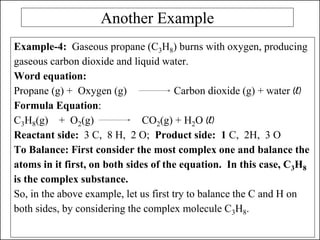
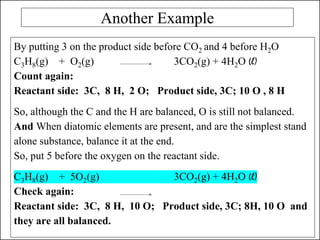
This document discusses chemical reactions and how to write and balance chemical equations. It defines chemical reactions as when new substances are formed with new properties, whereas physical changes only alter physical properties. To identify if a reaction occurred, one looks for evidence like new colors, gas formation, or heat/light production. Chemical equations represent reactions, with reactants on the left and products on the right. Formulas replace word names and physical states are indicated. Equations are balanced by adding coefficients to ensure equal numbers of each type of atom/ion on both sides of the equation. Several examples demonstrate how to write word equations, convert to formulas, and balance chemical equations.