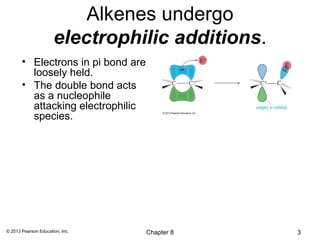
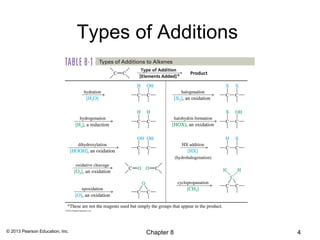
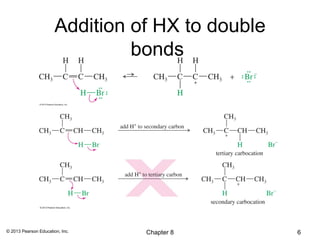
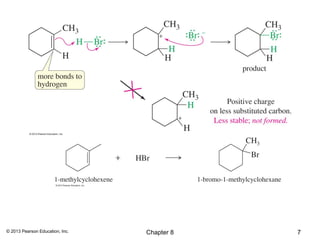
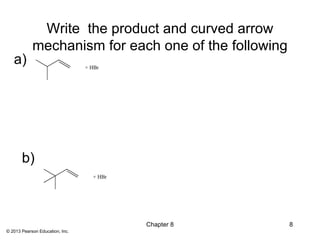
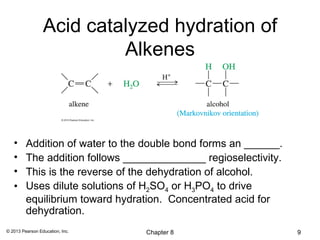
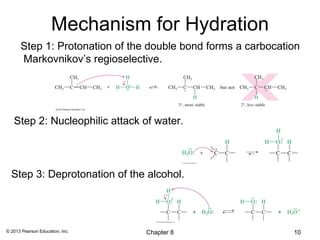
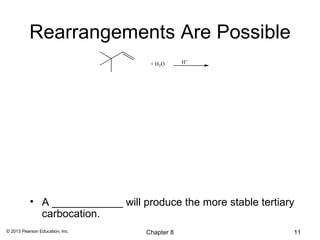
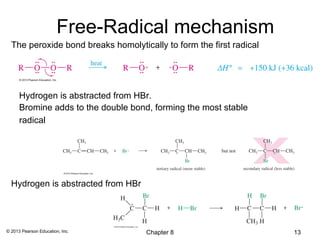
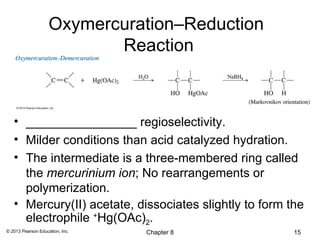
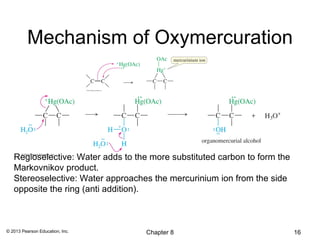
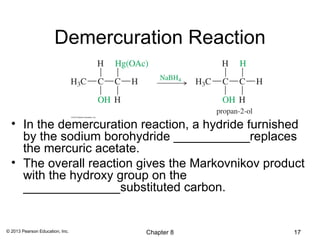
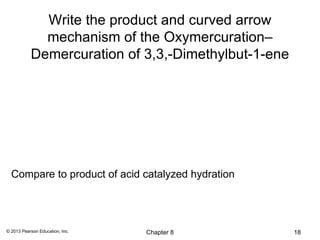
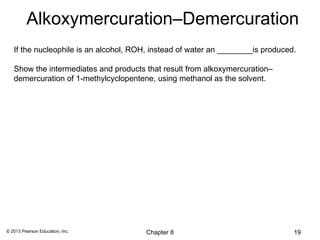
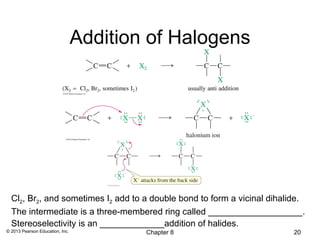
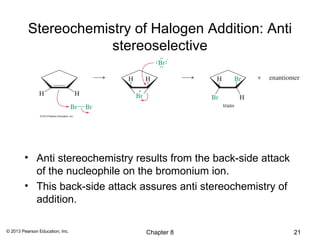
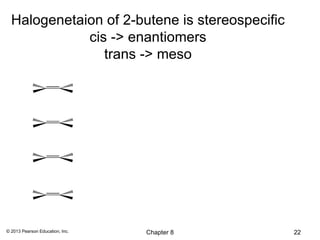
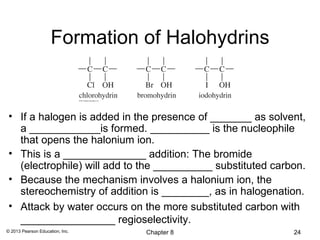
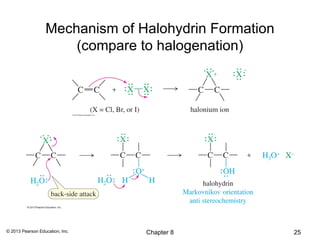
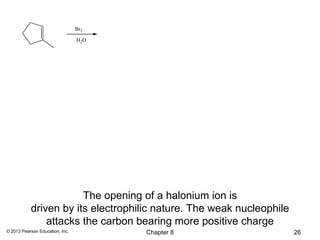
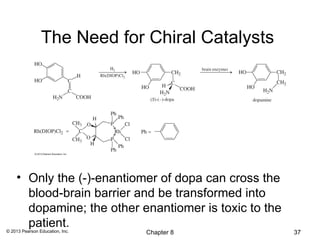
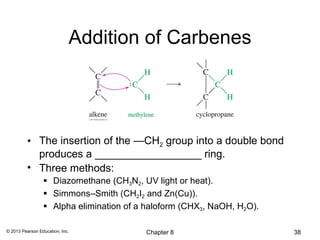
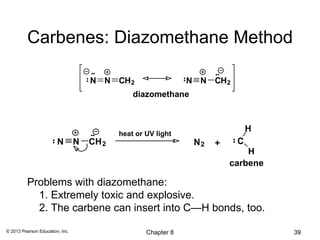
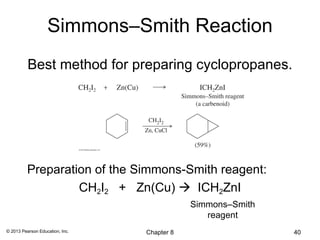
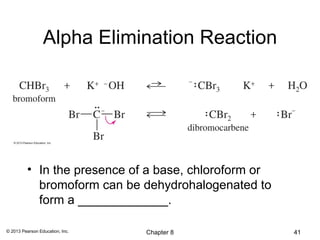
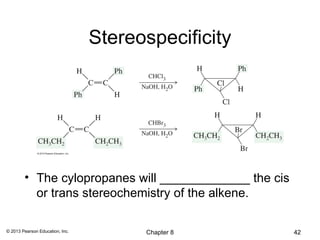
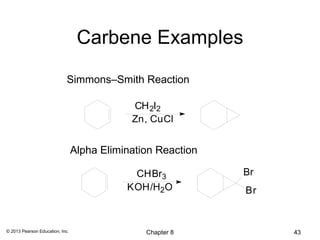
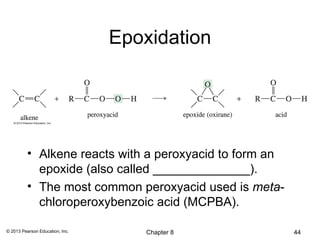
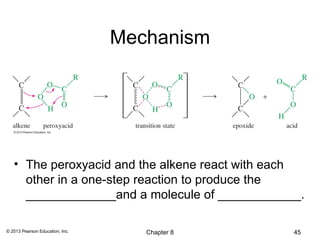
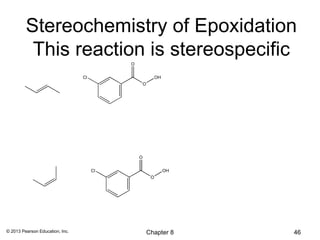
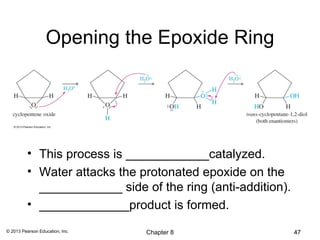
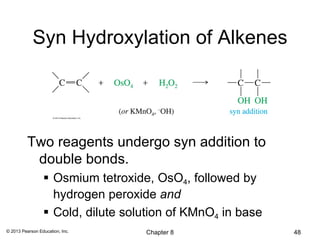
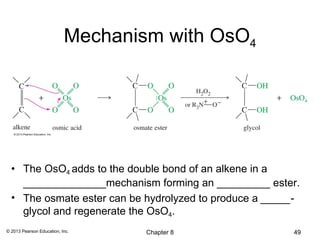
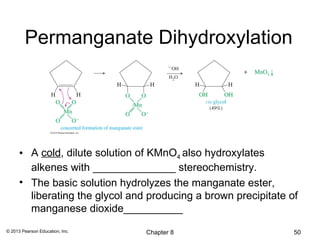
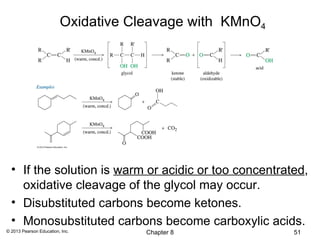
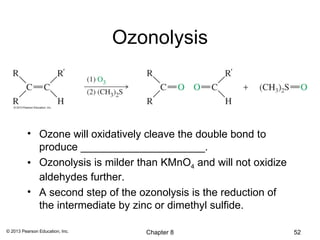
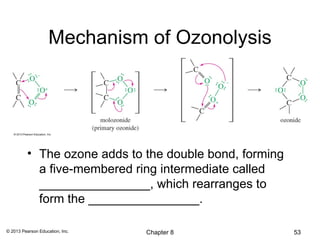
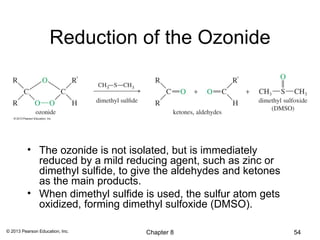
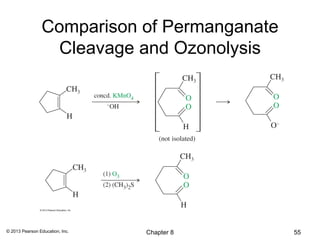
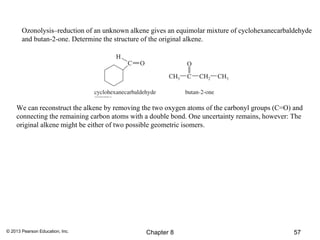
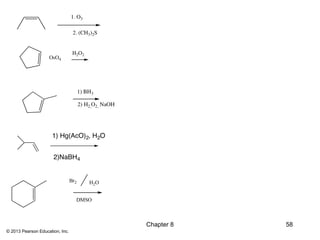
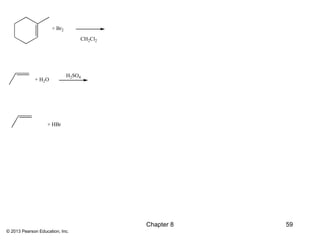
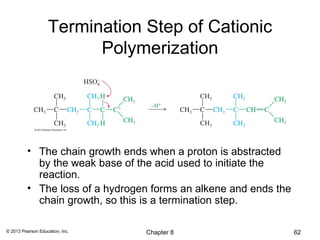
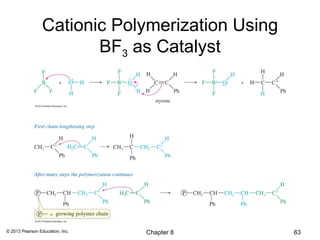
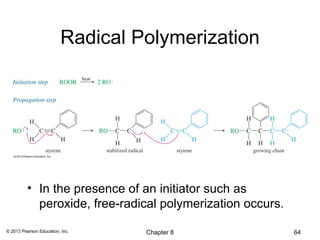
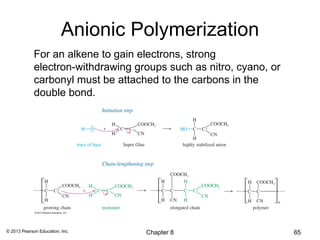
1) Alkenes undergo electrophilic addition reactions with reagents such as HX, H2O, X2, Br2/H2O, H2, carbenes, and peroxyacids. The double bond acts as a nucleophile, attacking electrophilic species.
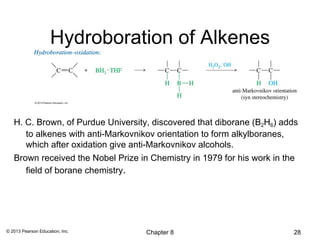
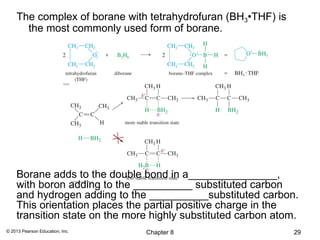
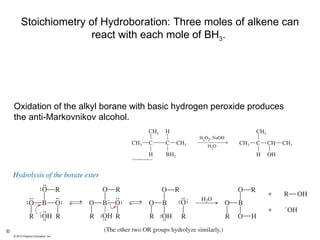
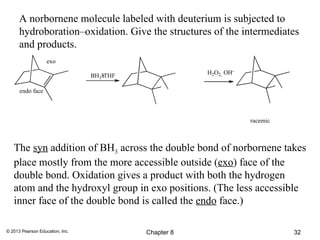
2) Hydroboration-oxidation adds BH3 across the double bond in an anti-Markovnikov fashion, forming an alkylborane. Oxidation of the alkylborane produces the anti-Markovnikov alcohol.
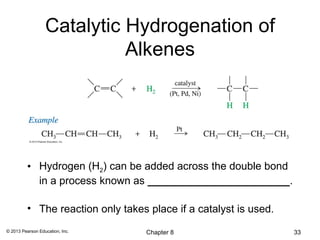
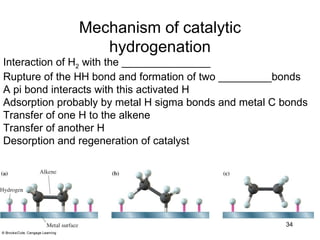
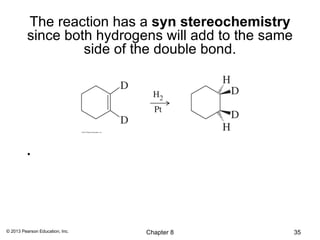
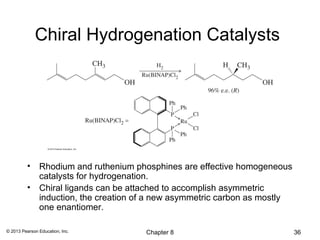
3) Catalytic hydrogenation adds H2 across the double bond in a syn fashion using a metal catalyst such as rhodium or ruthenium