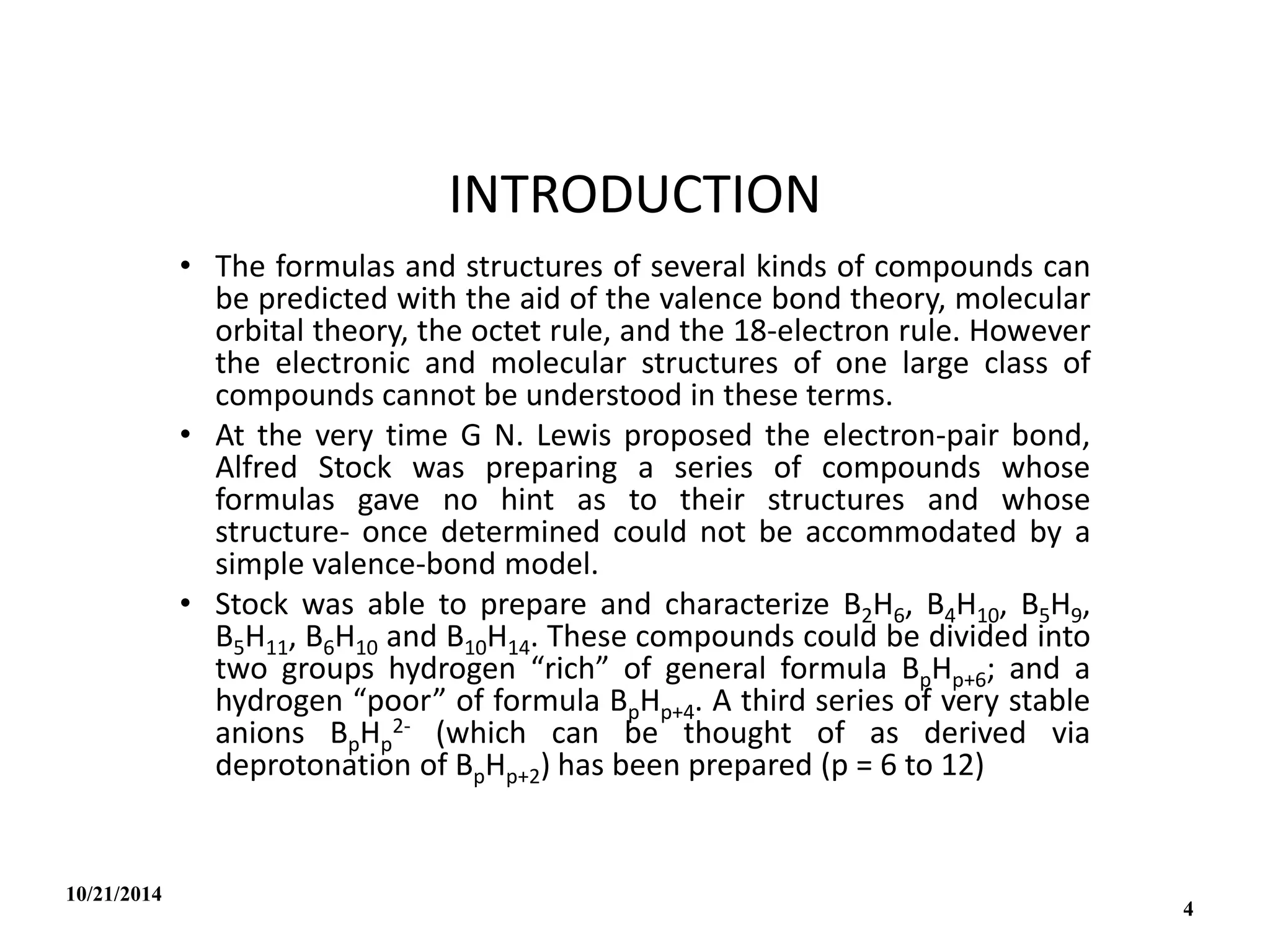
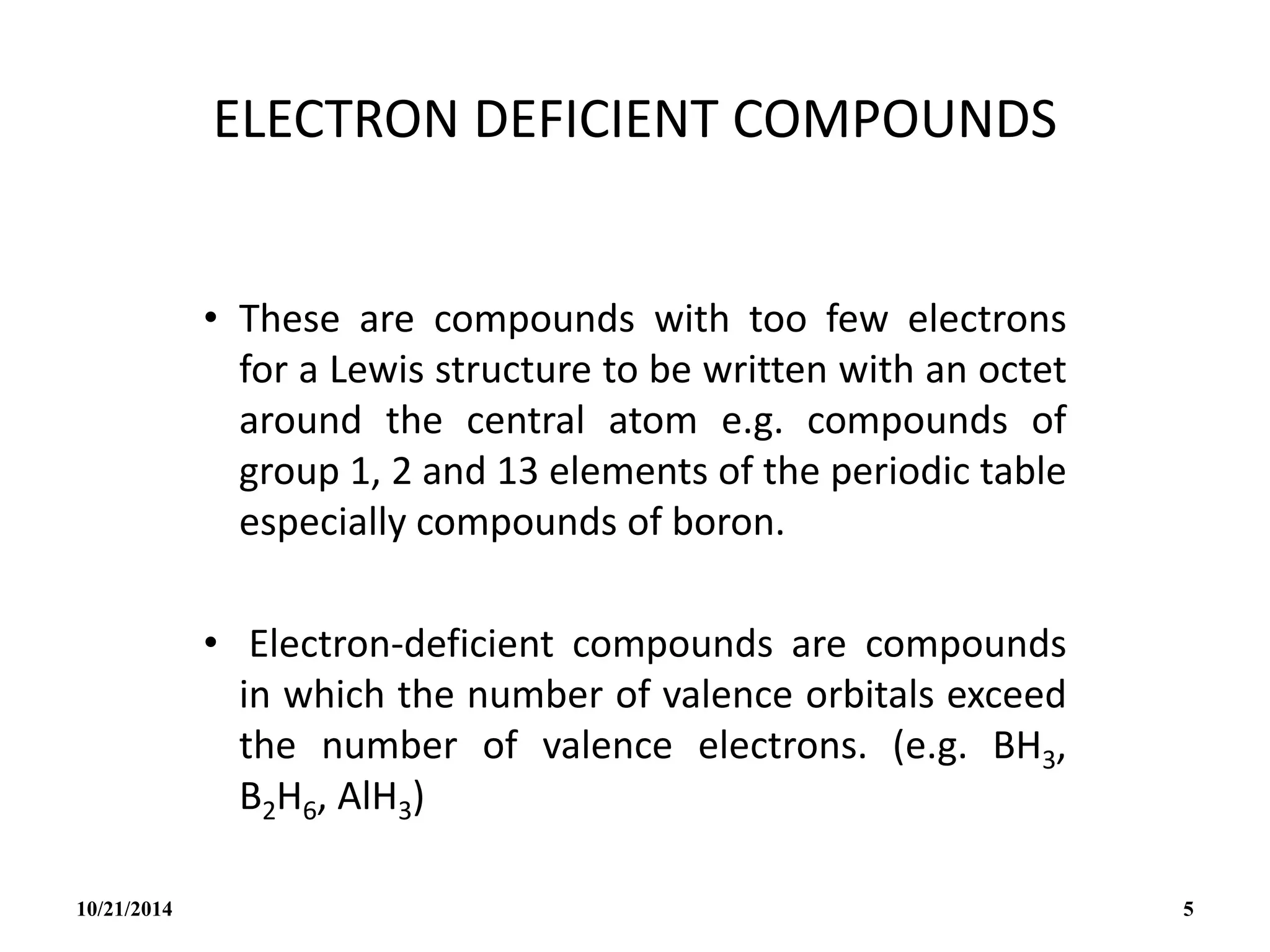
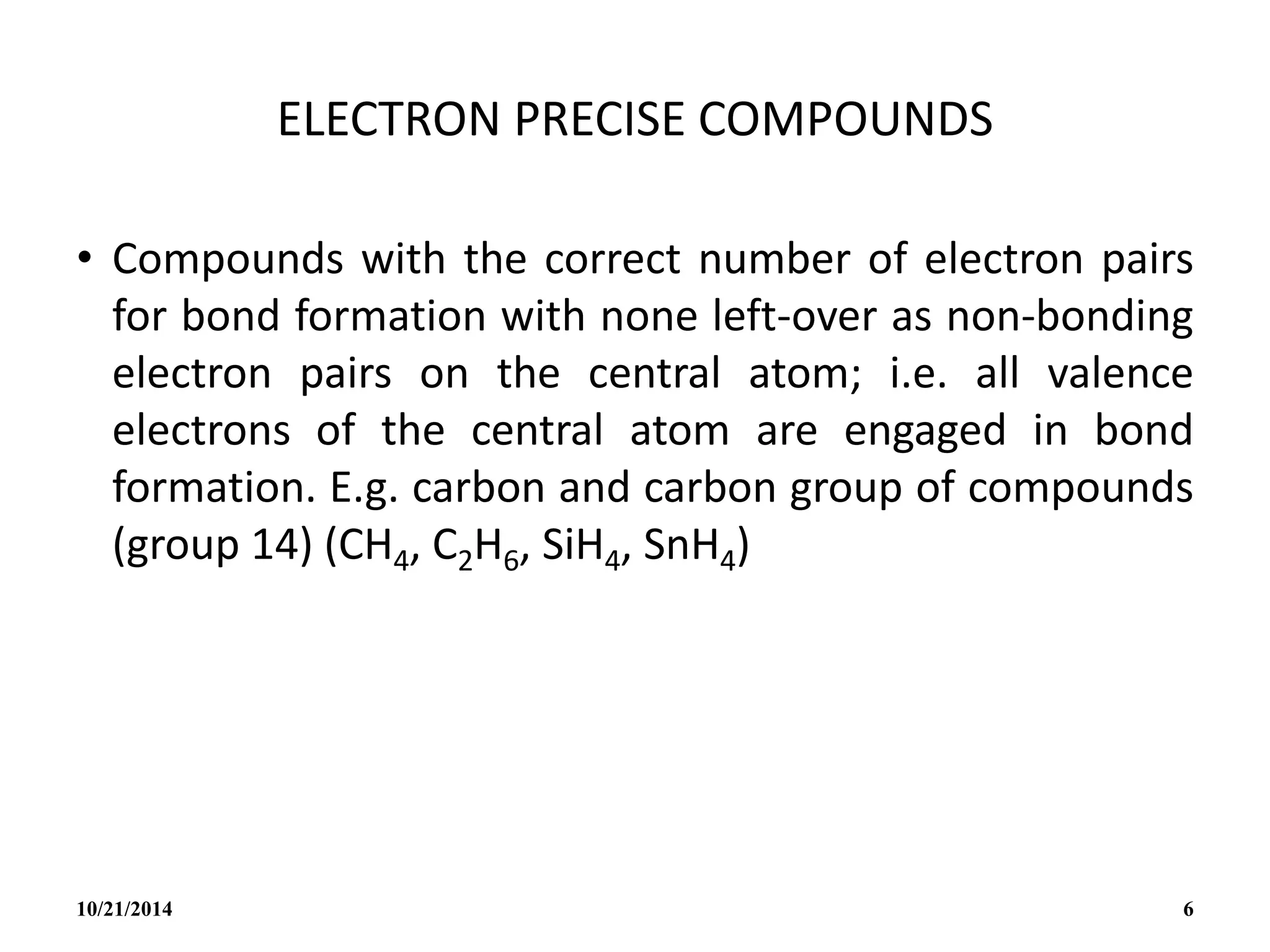
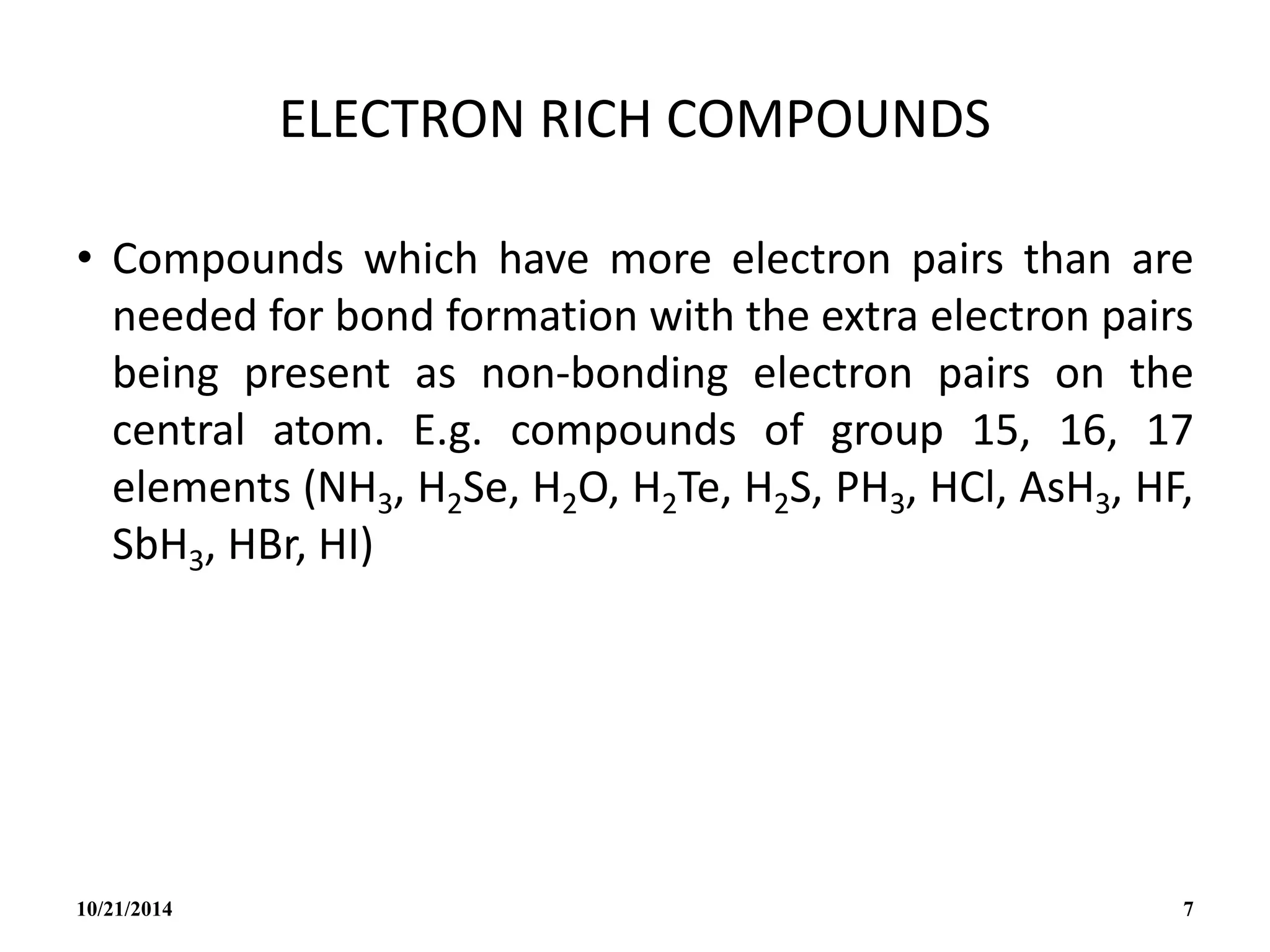
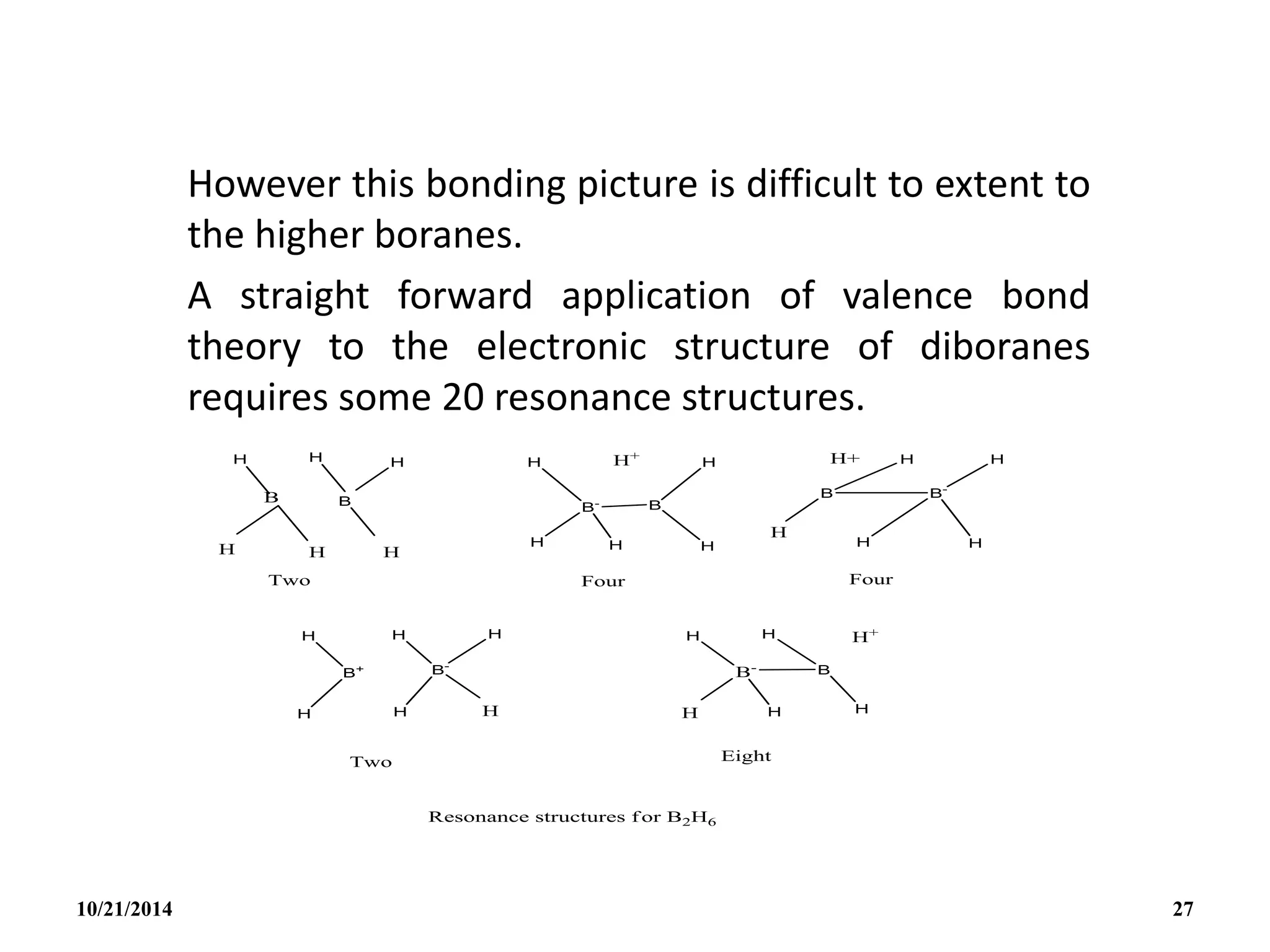
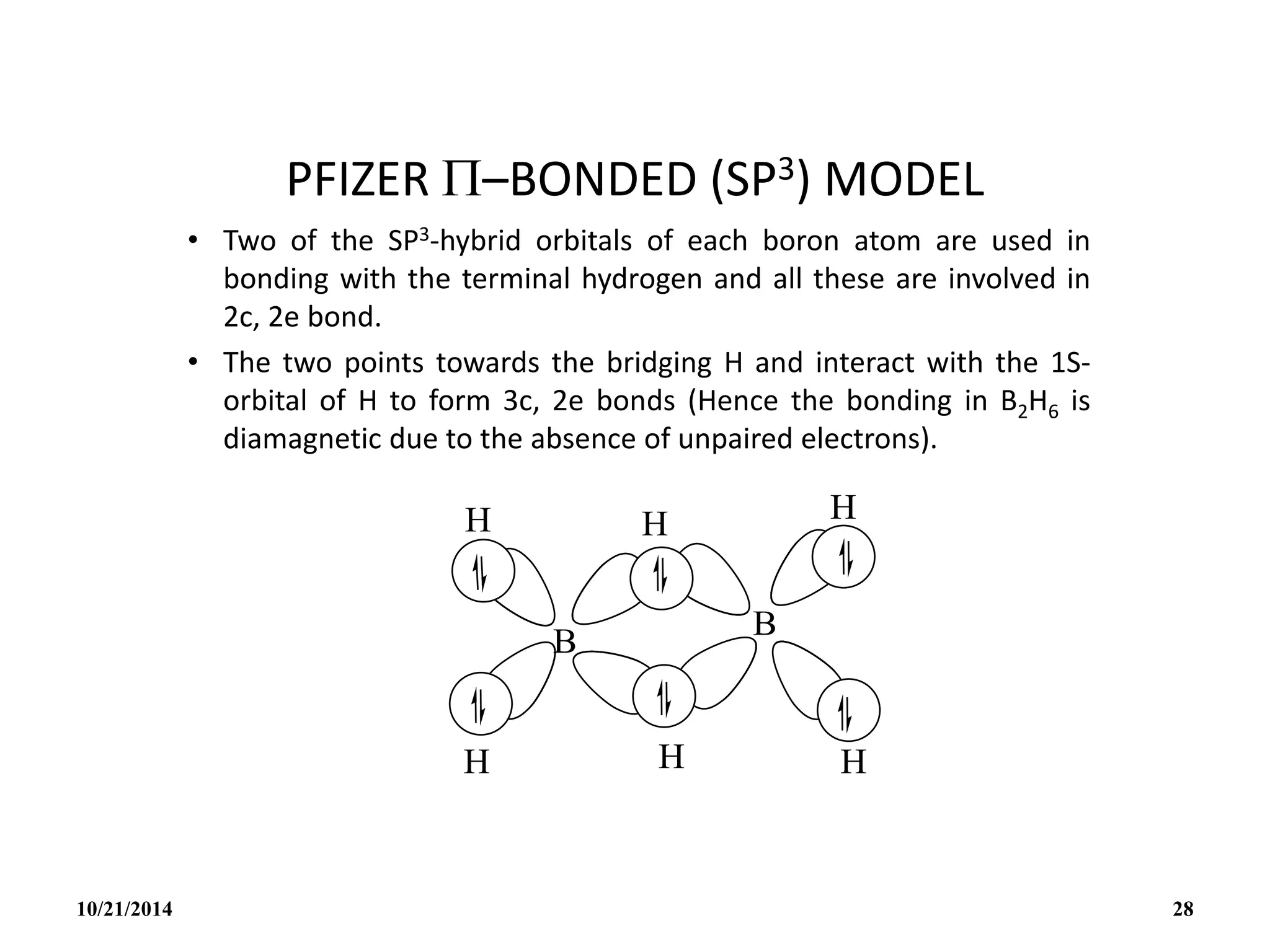
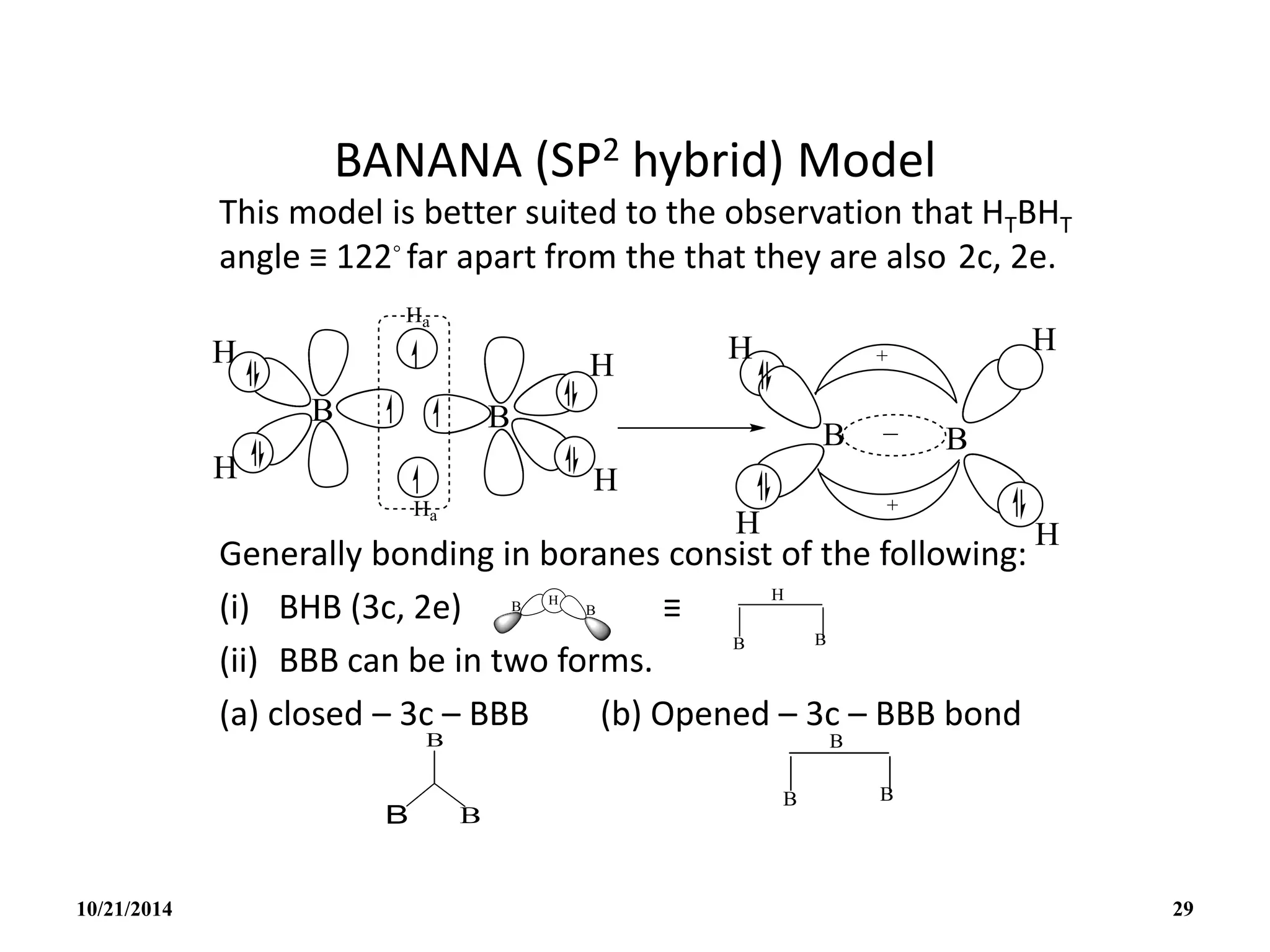
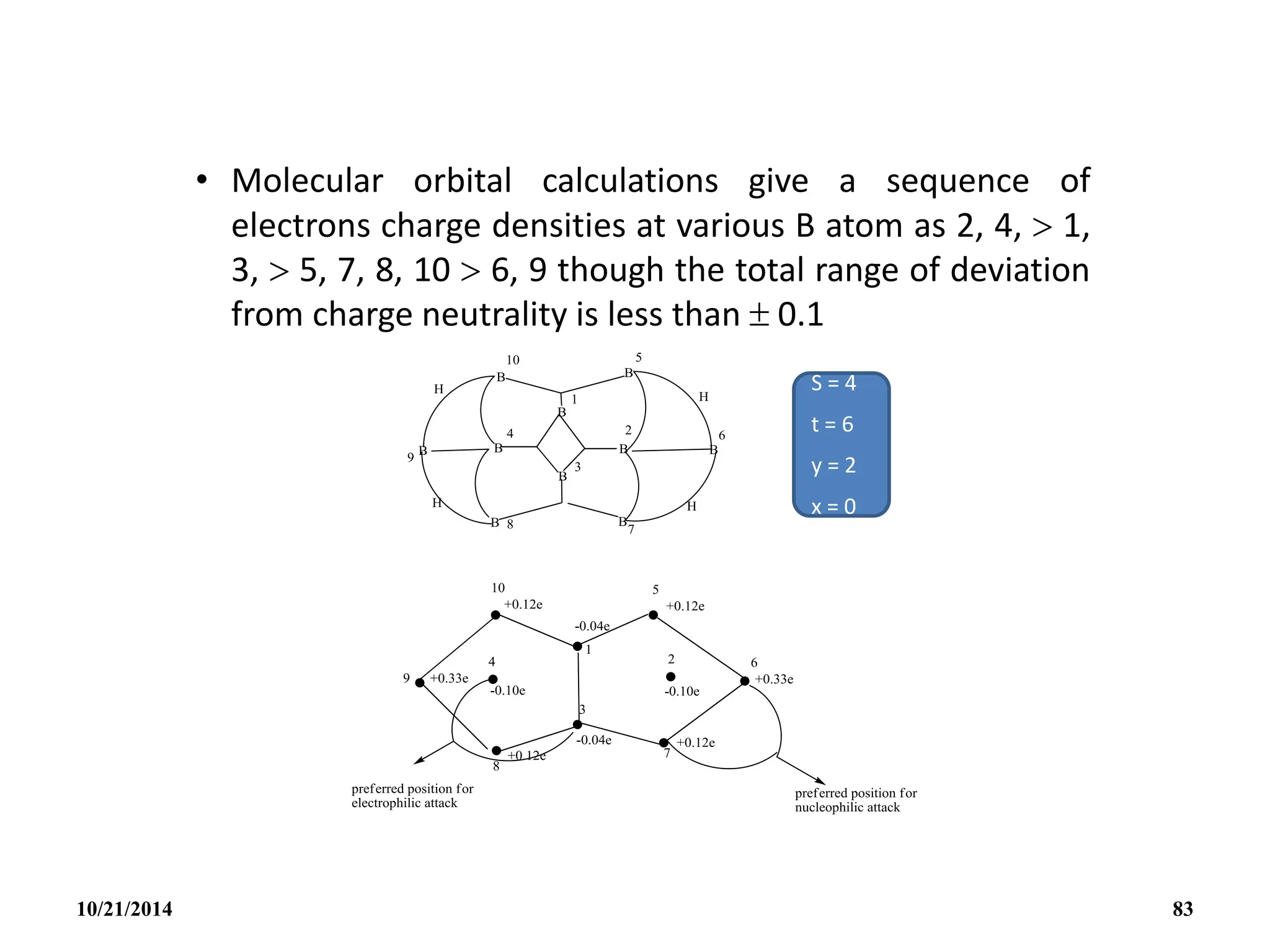
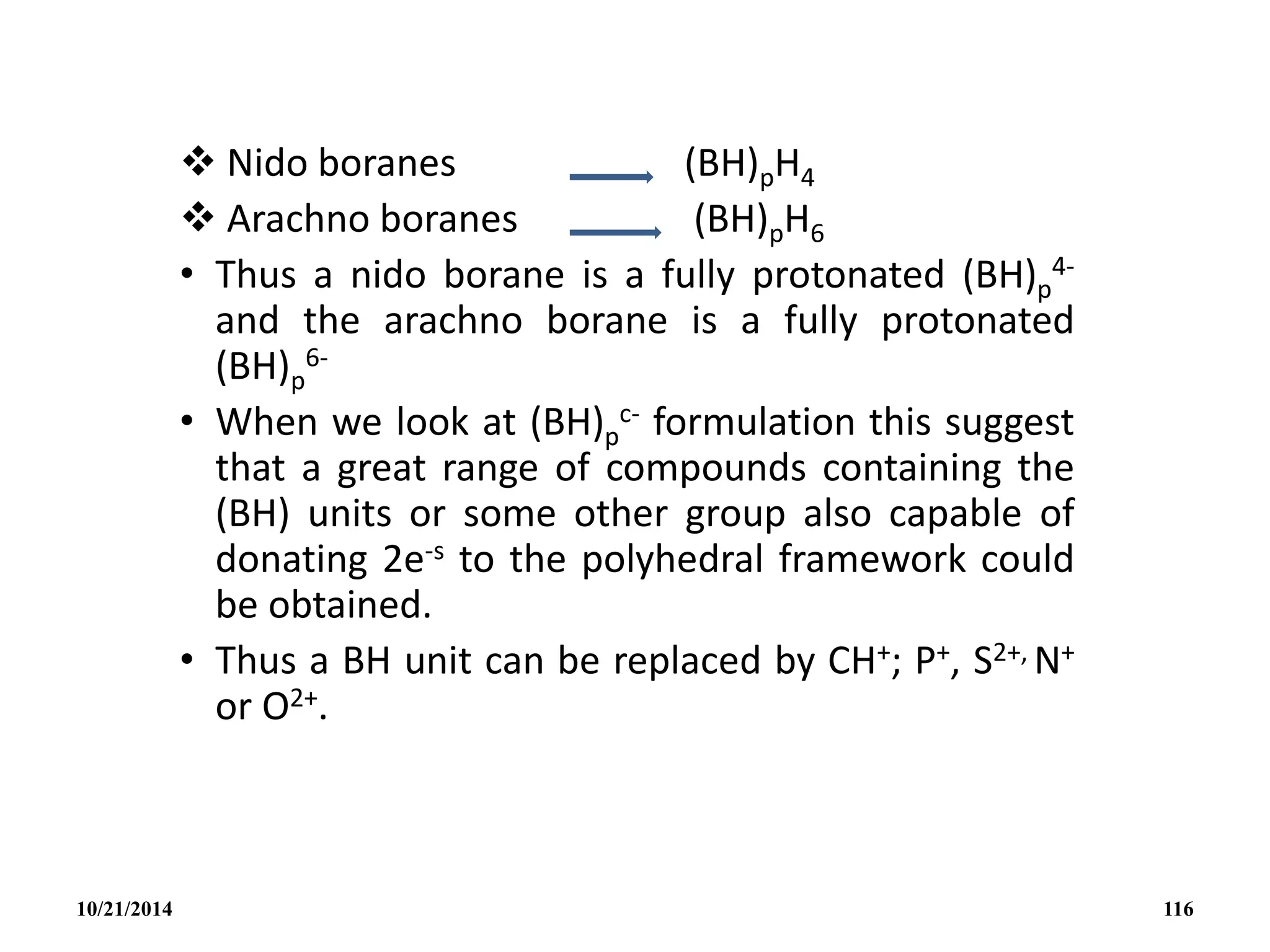
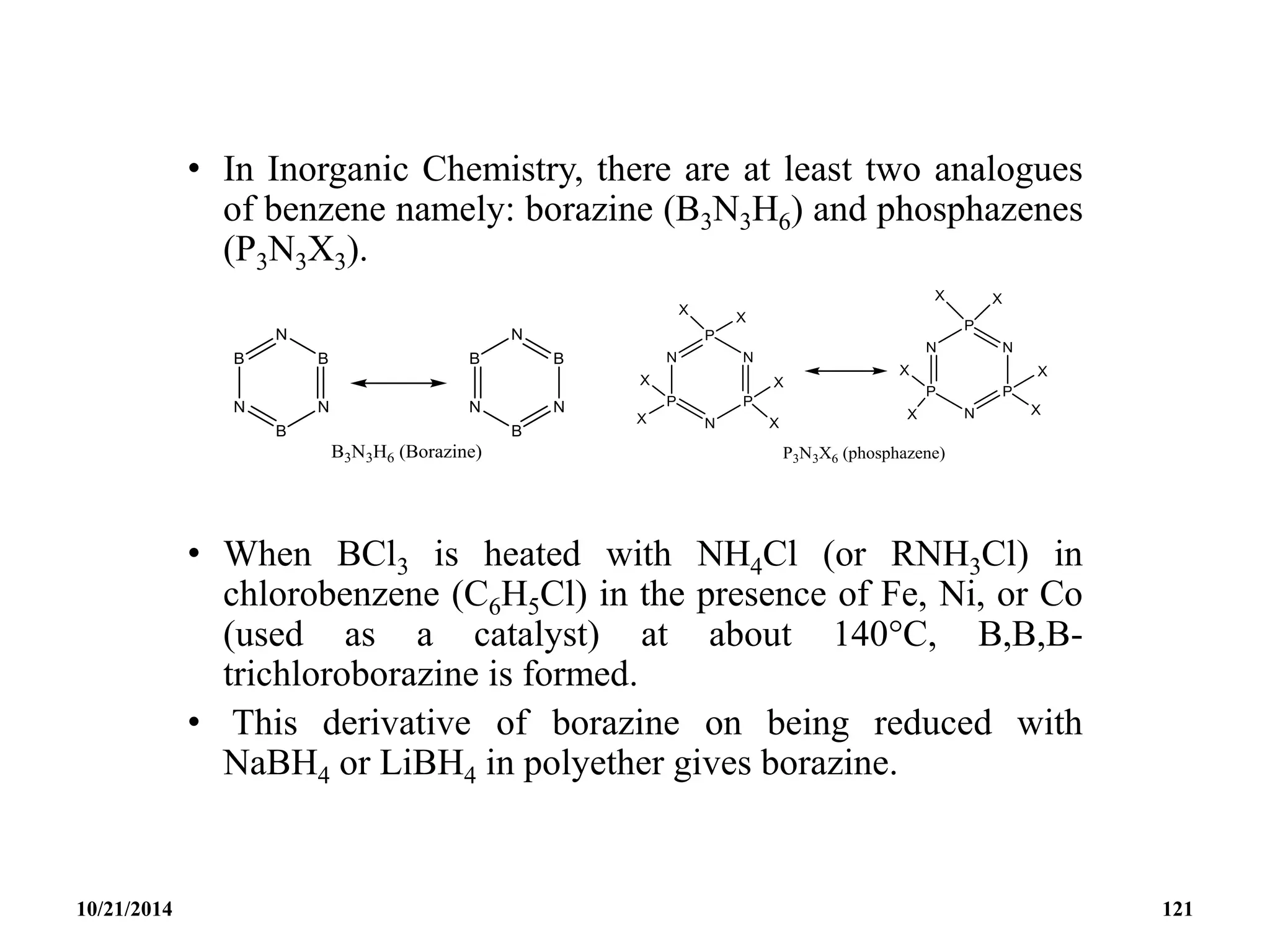
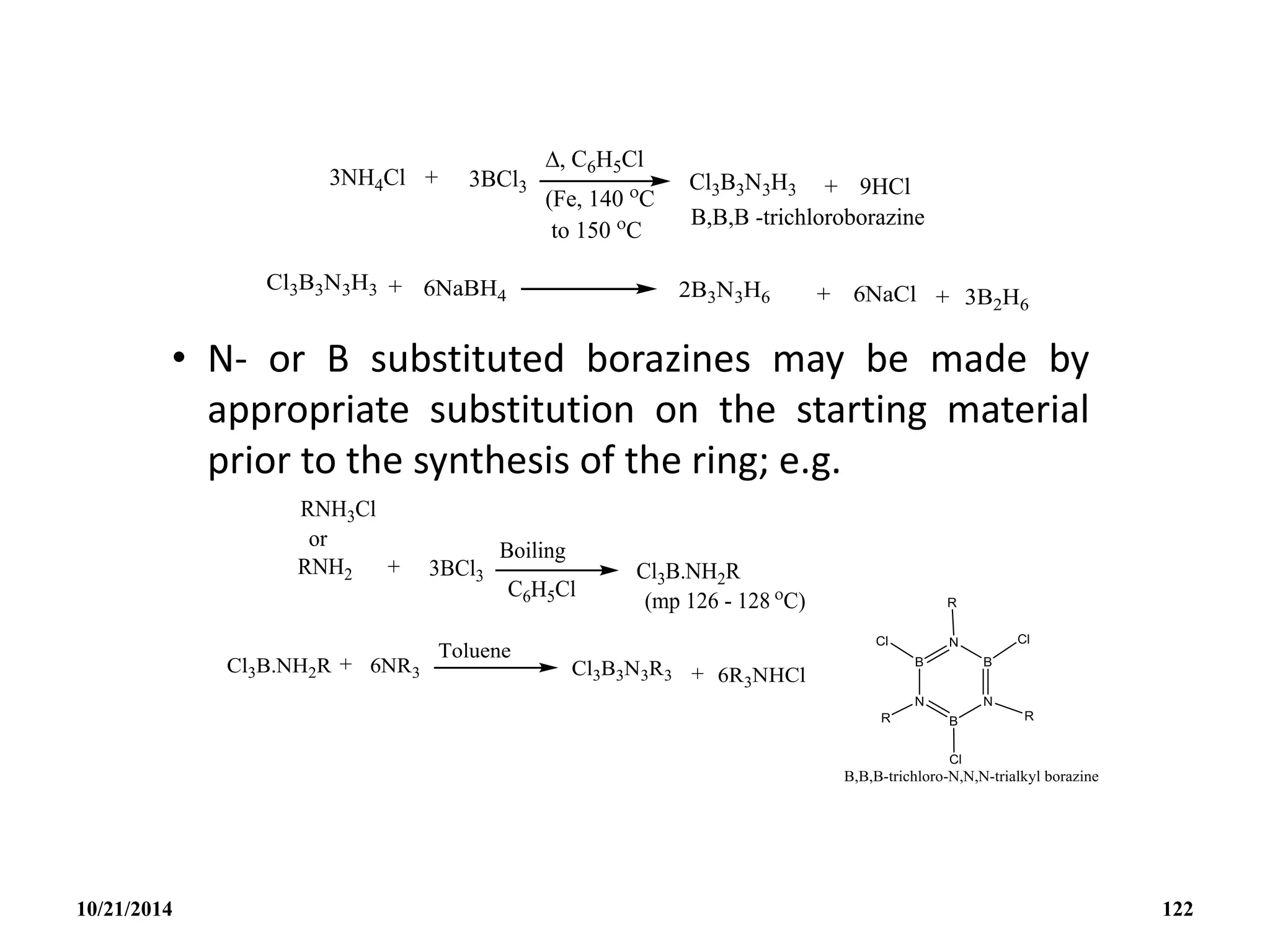
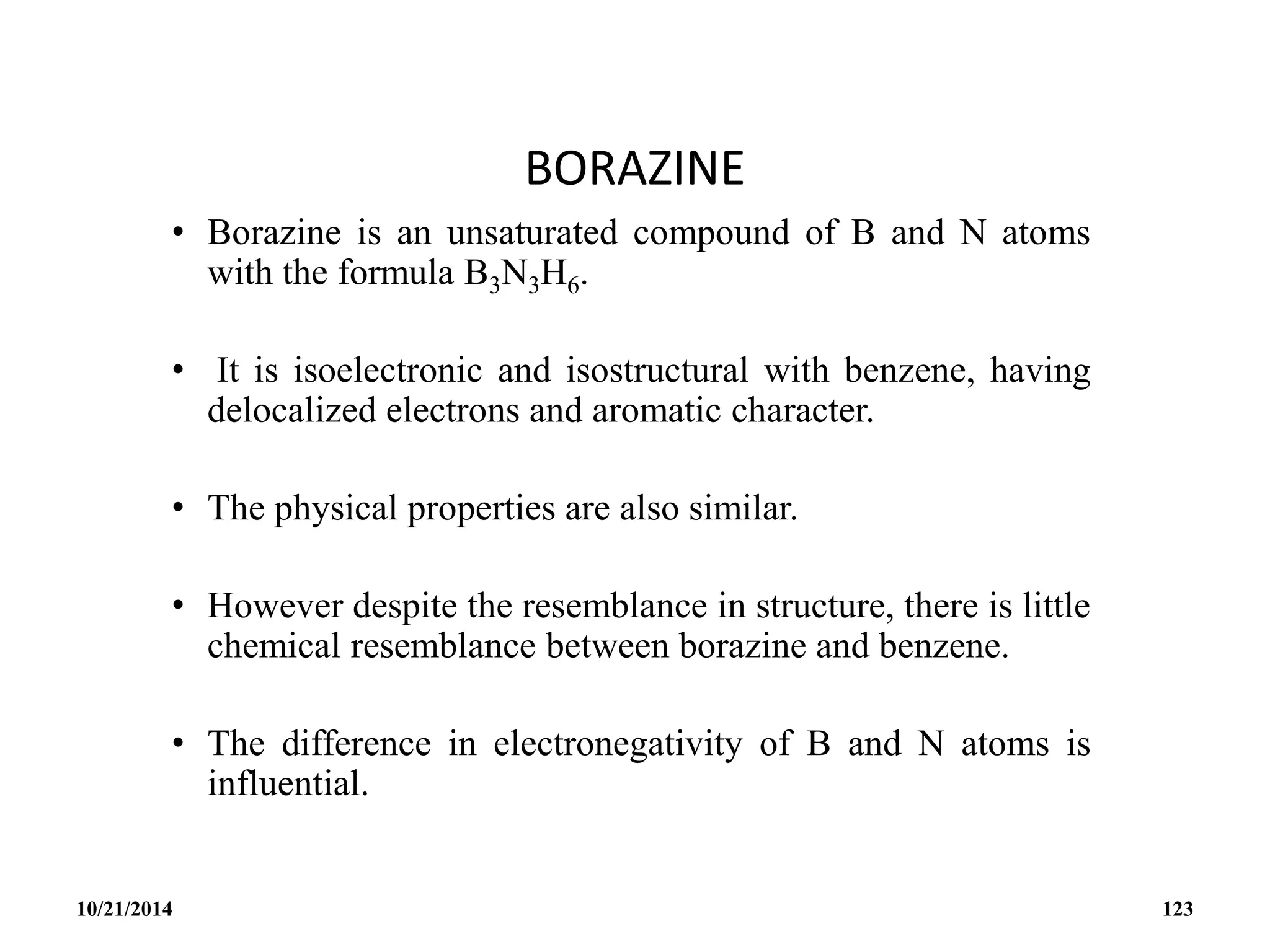
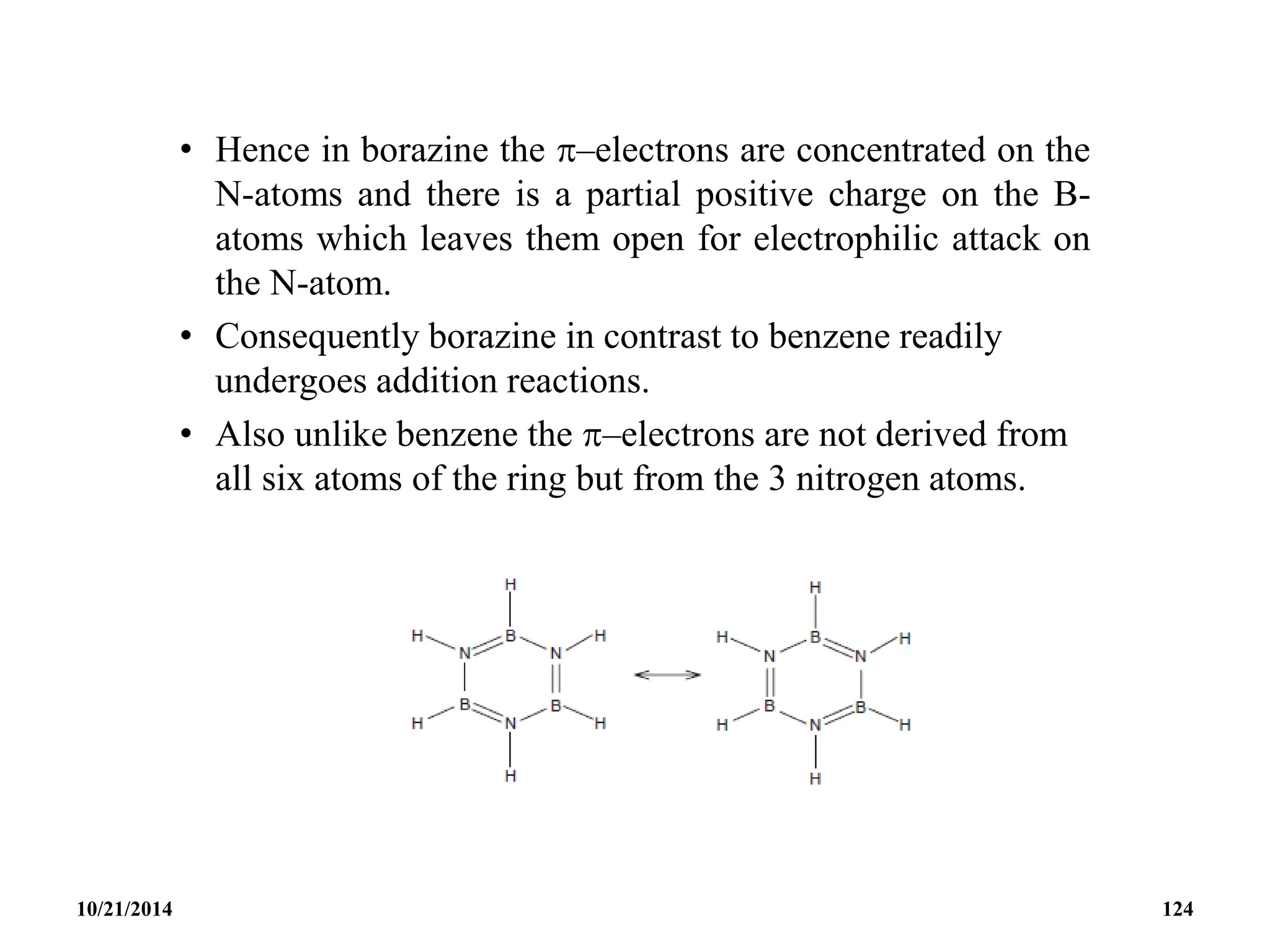
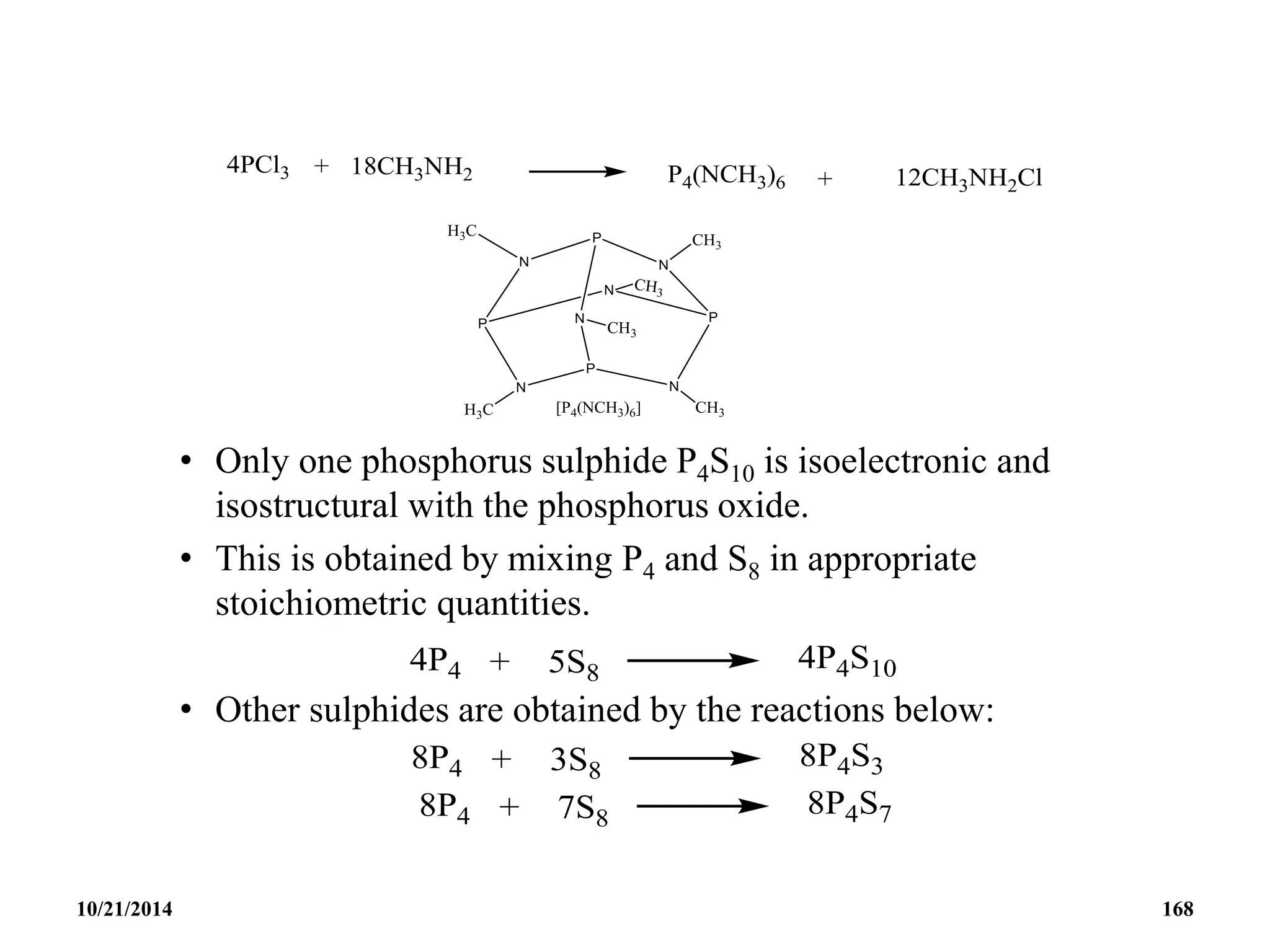
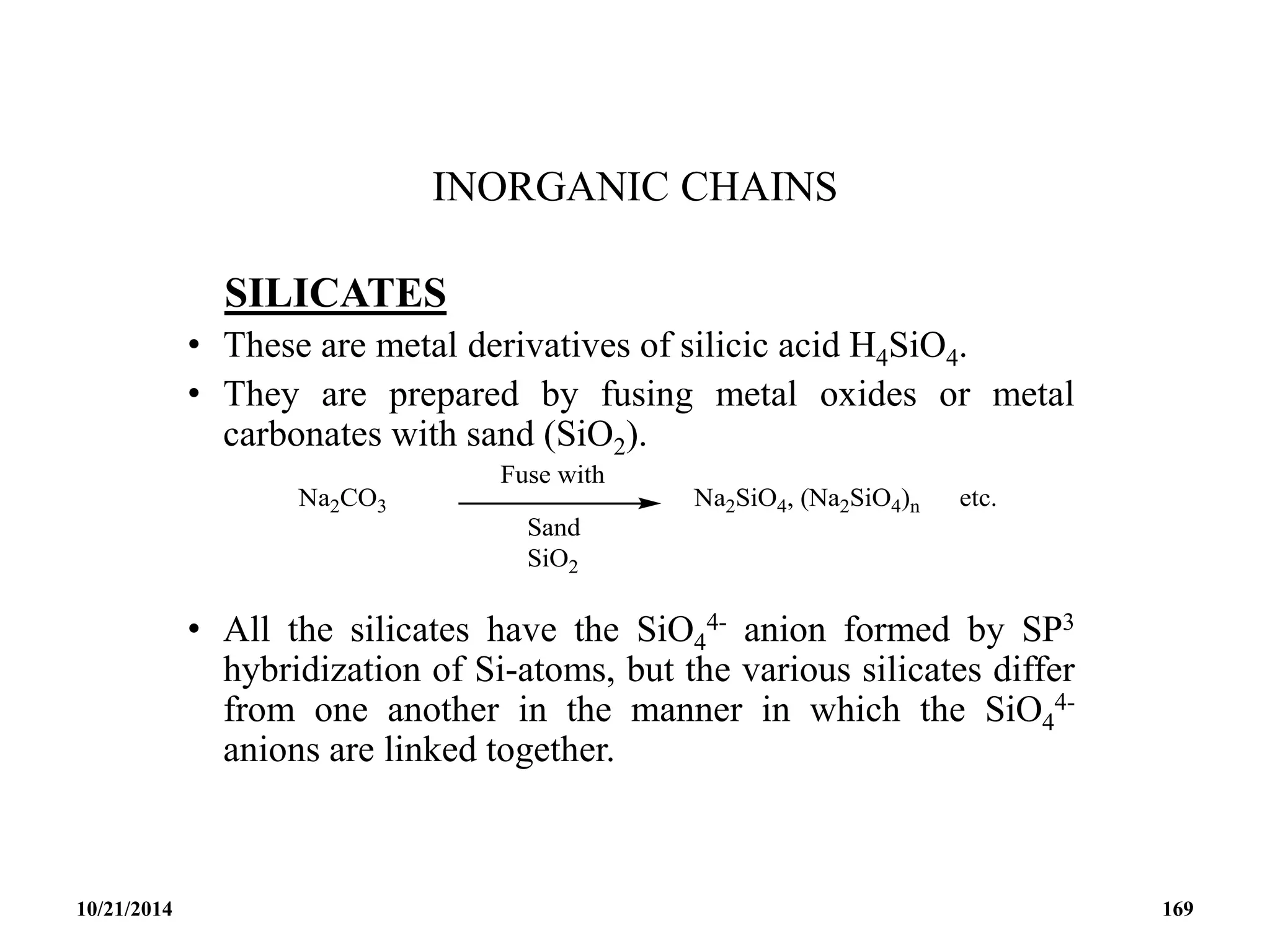
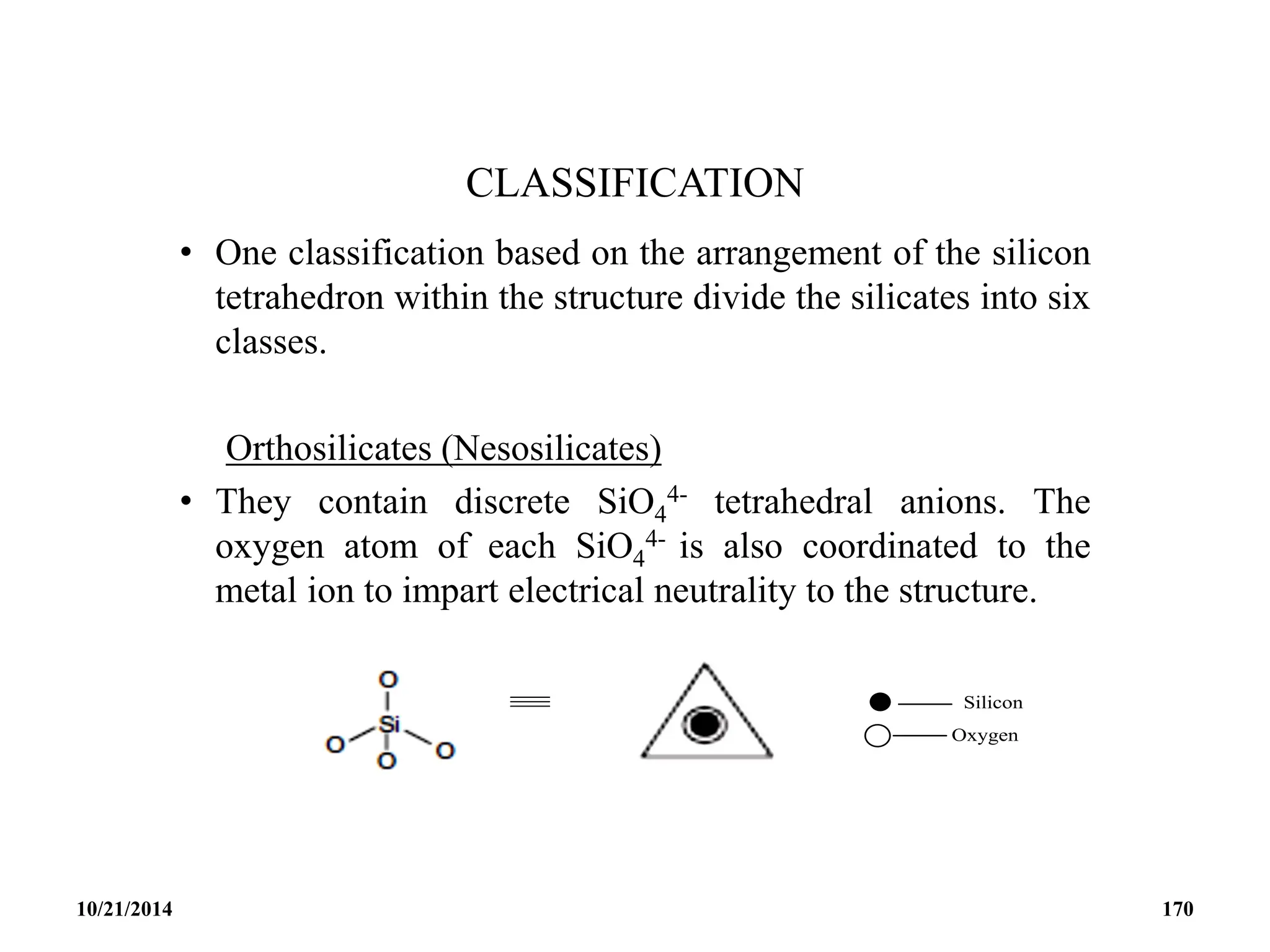
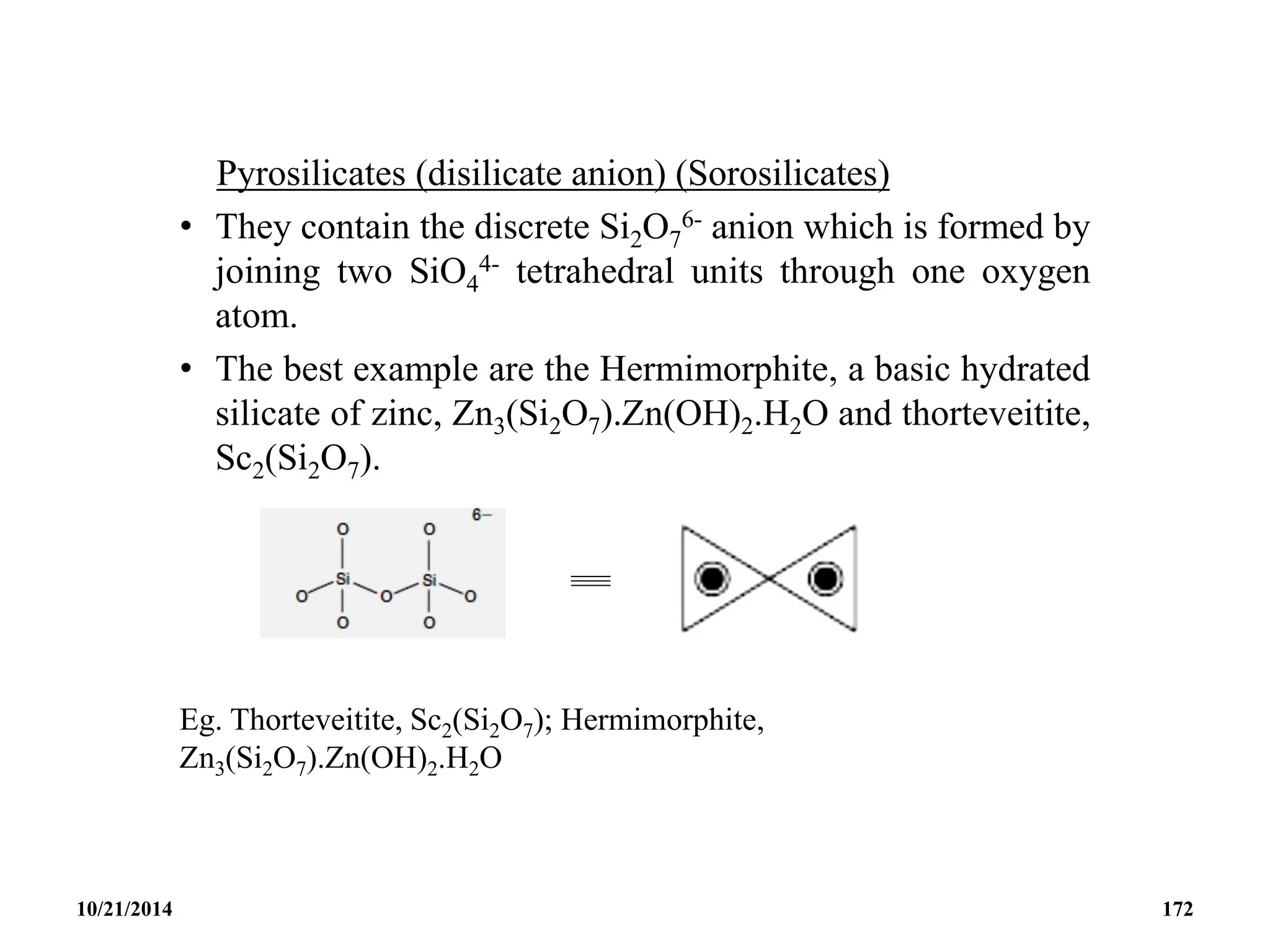
This document outlines a course on inorganic polymers and electron deficient compounds. It discusses various types of electron deficient compounds such as boron compounds. It also covers inorganic polymers, rings, cages and silicates. The document provides references for further reading. It introduces boranes and describes their classification using Wade's rule. It discusses various borane structures such as closo, nido and arachno, and provides examples. It also covers borane nomenclature, bonding models including molecular orbital theory, and summarizes the key points about boranes and their classification.



![WADE’S RULE
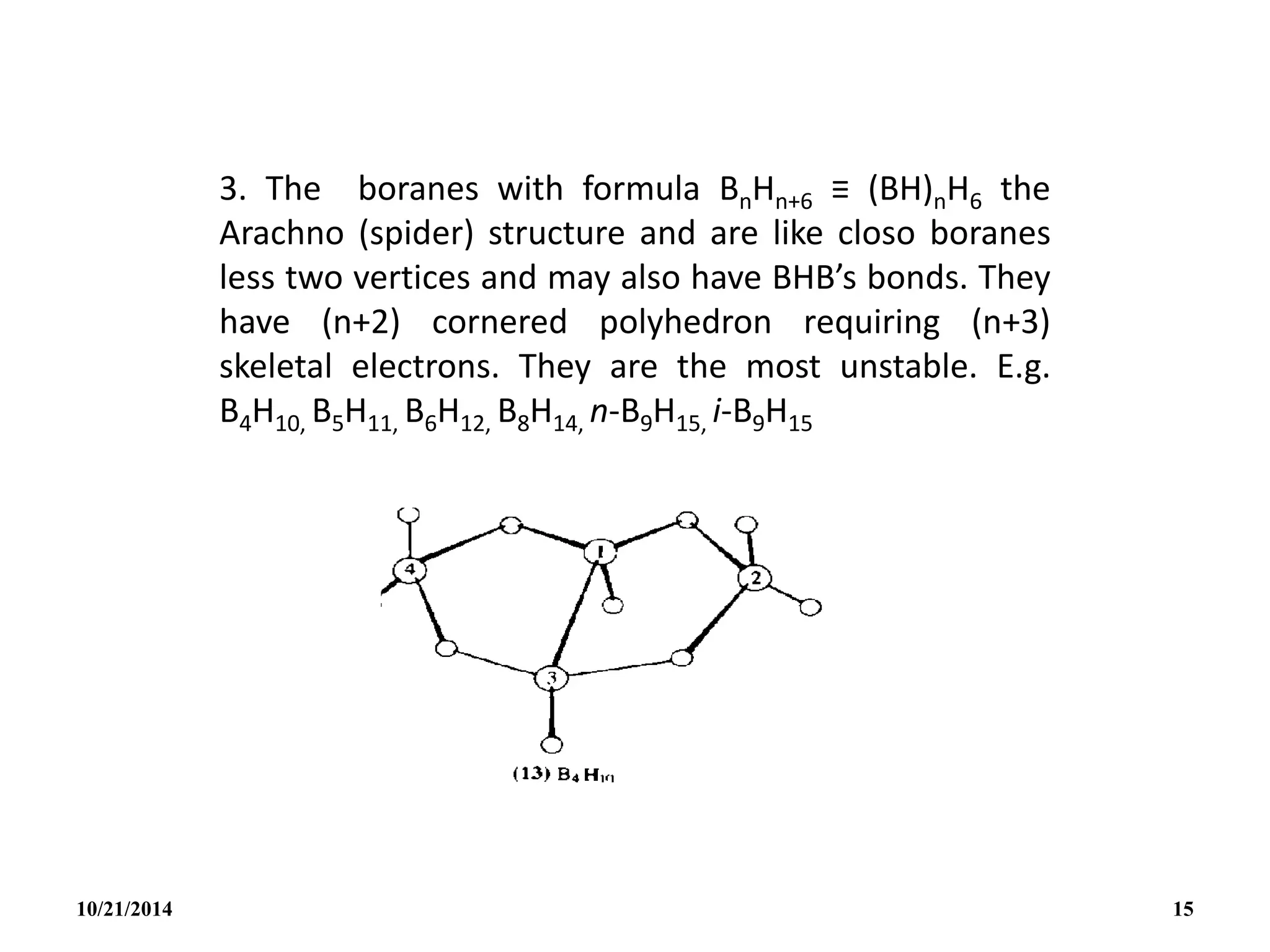
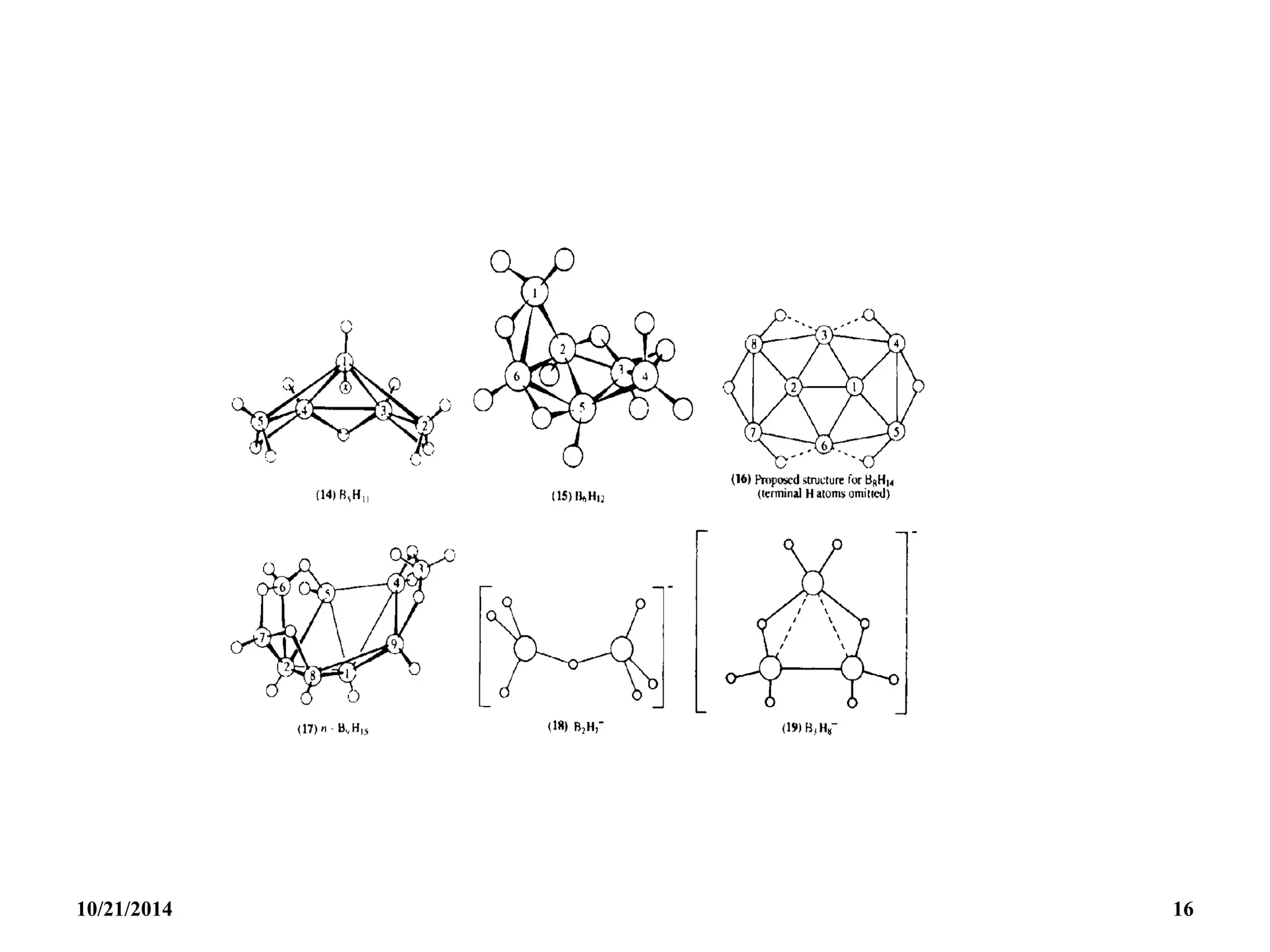
1. Boranes of formula [BnHn]2- will be found to have the
CLOSO (caged) structure with a B unit at each corner
of a closed deltahedron and no BHB bonds in the
closo structure.
Such structure are known to have (n+1) skeletal
electrons.
These series of anions is known for n = 5 to 12
Trigonalbipyramidal [B5H5]2-
Octahedral [B6H6]2-
Icosahedral [B12H12]2-
NB: The closo hydroborates and carboranes are often
thermally stable and fairly unreactive.
10/21/2014 11](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-11-2048.jpg)

![SUMMARY
TYPE FORMULA SKELETAL
ELECTRON PAIRS
CORNERS OF
POKLYHEDRON
EXAMPLES
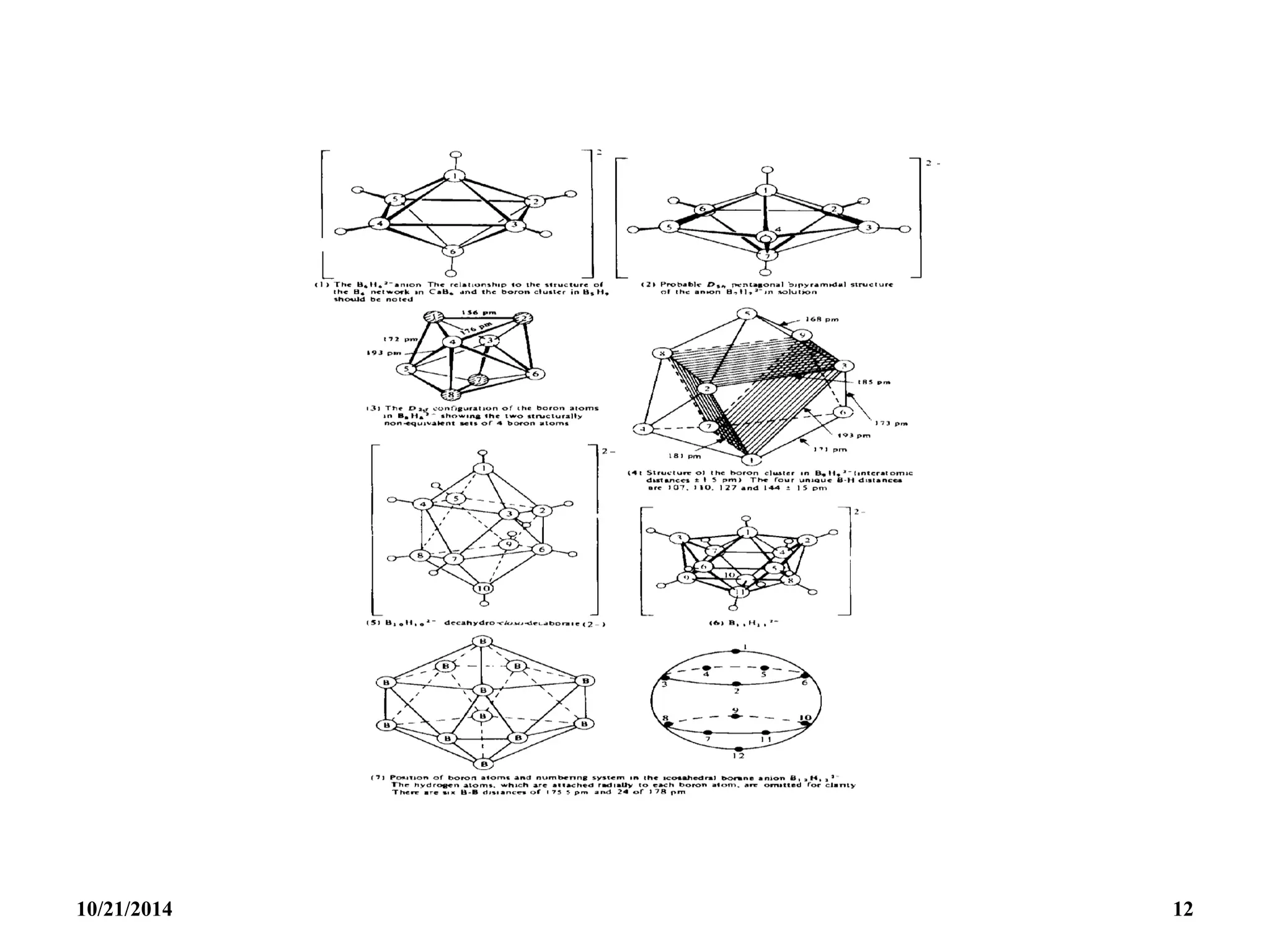
Closo [BnHn]2- n+1 n [BnHn]2- to[B12H12]2-
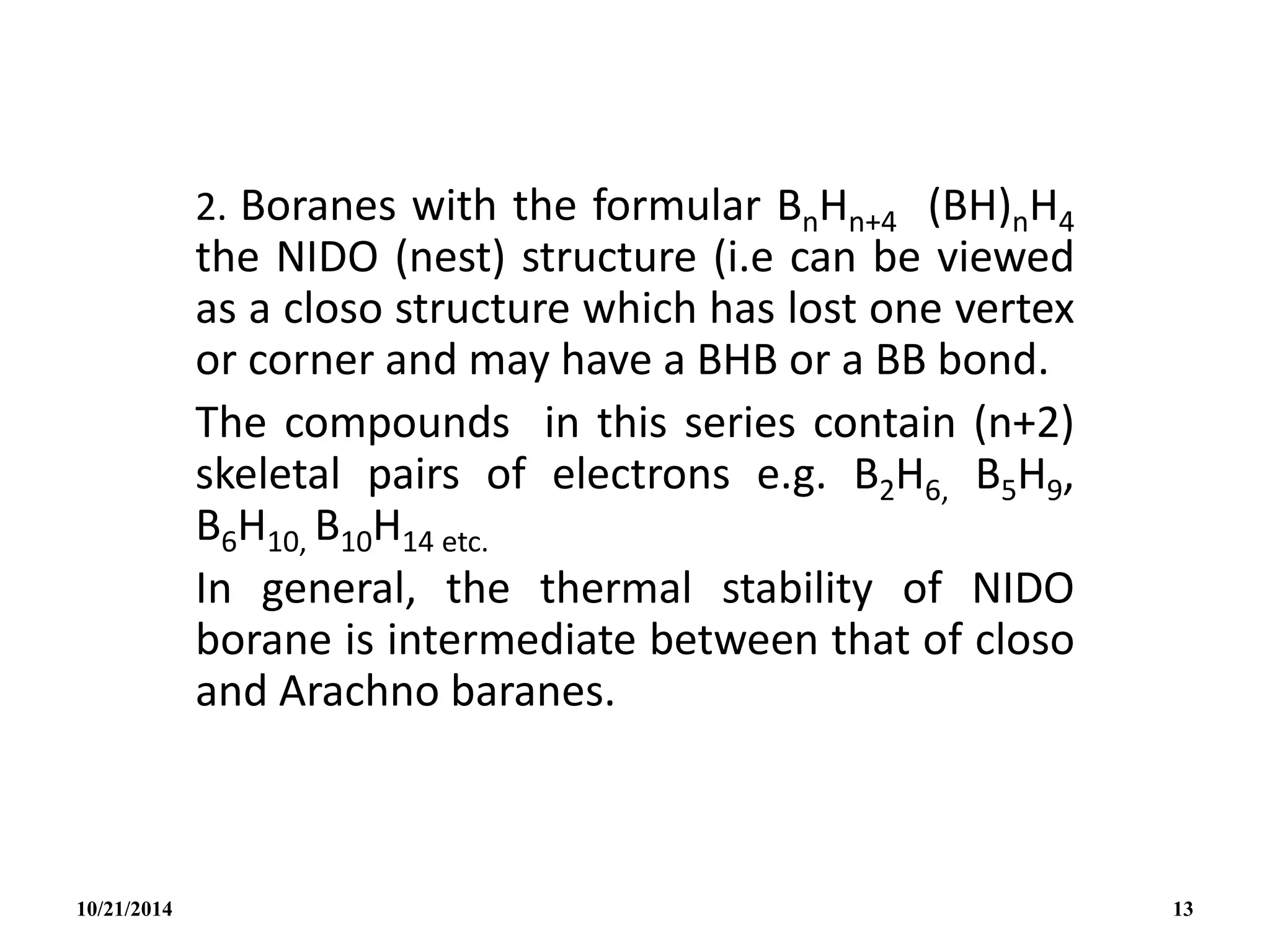
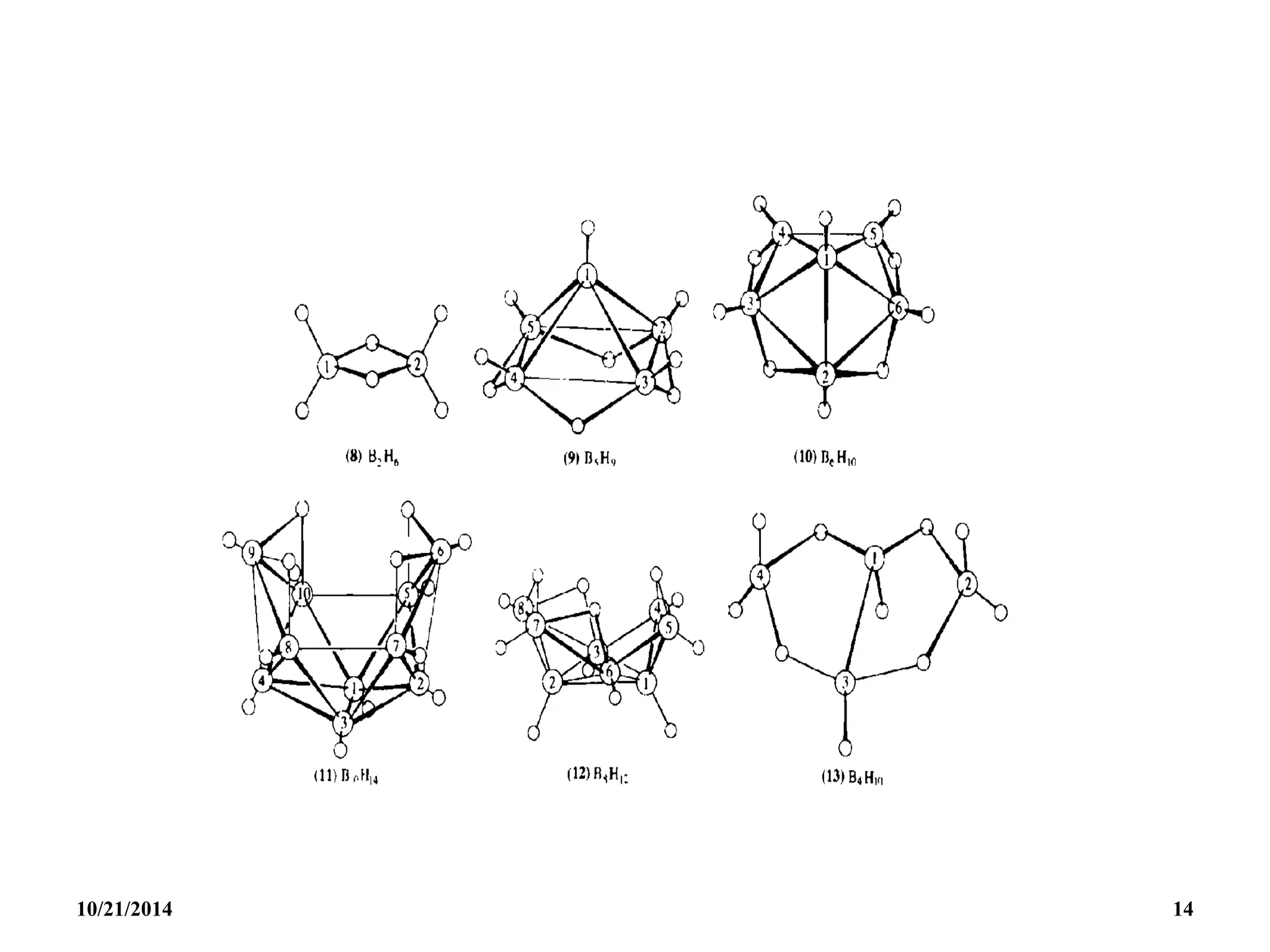
Nido BnHn+4 n+2 n + 1 B2H6, B2H6, B2H6
Arachno BnHn+6 n+3 n + 2 B4H10, B5H11
Hypho BnHn+8 n+4 n + 3 B8H16, B10H18
Klado BnHn + 10 n+5 n + 4
Conjuncto BnH m B8H18, B15H23 etc.
10/21/2014 20](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-20-2048.jpg)
![Using Wades Rule
E.g.
(i) [B5H5]2- Closo structure
5(B-H) 5 x 2e = 10e-s
overall charge 2e- = 2e-s
(5+1)e- pairs 12e-s
i.e. From the formula [BnHn]2- with (n+1) pair skeletal
electrons
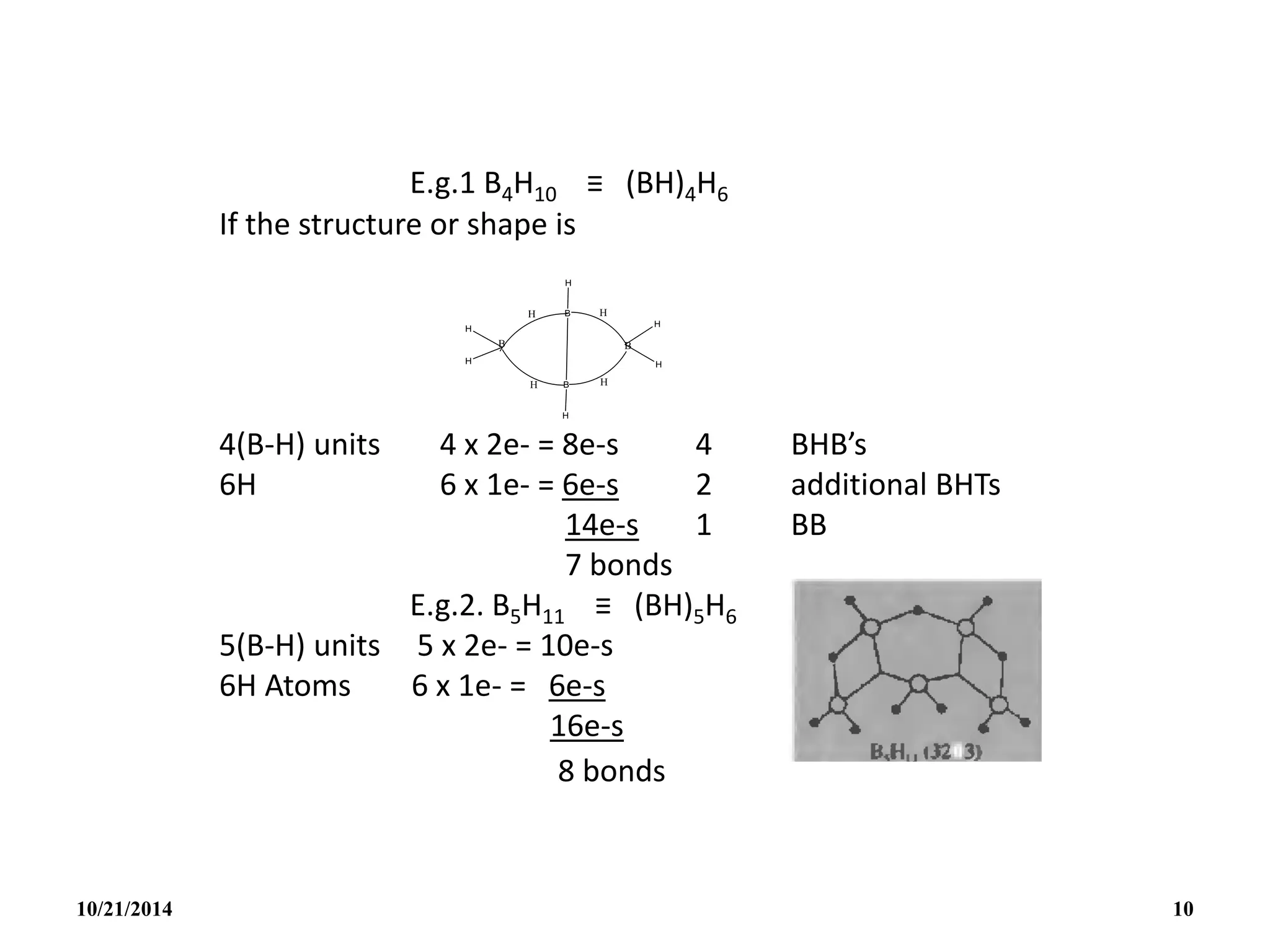
(ii) B5H9 ≡ (BH)5H4 Nido structure
5(B-H) 5 x 2e = 10e-s
4H 4 x 1e = 4e-s
overall charge 2e- = 2e-s
(5+2)e- pairs 14e-s
From BnHn+4 with n+2 skeletal electron pairs
10/21/2014 21](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-21-2048.jpg)
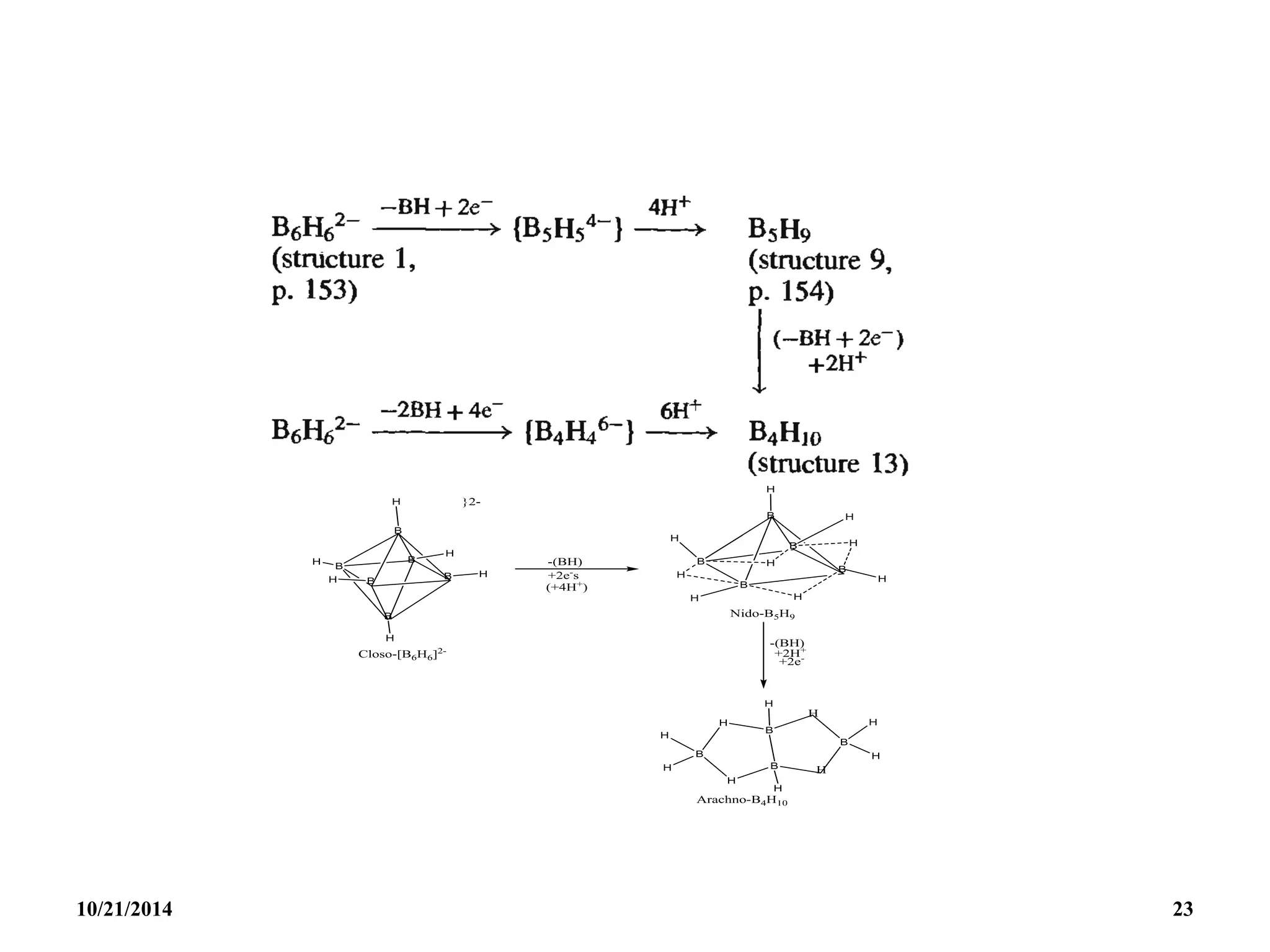
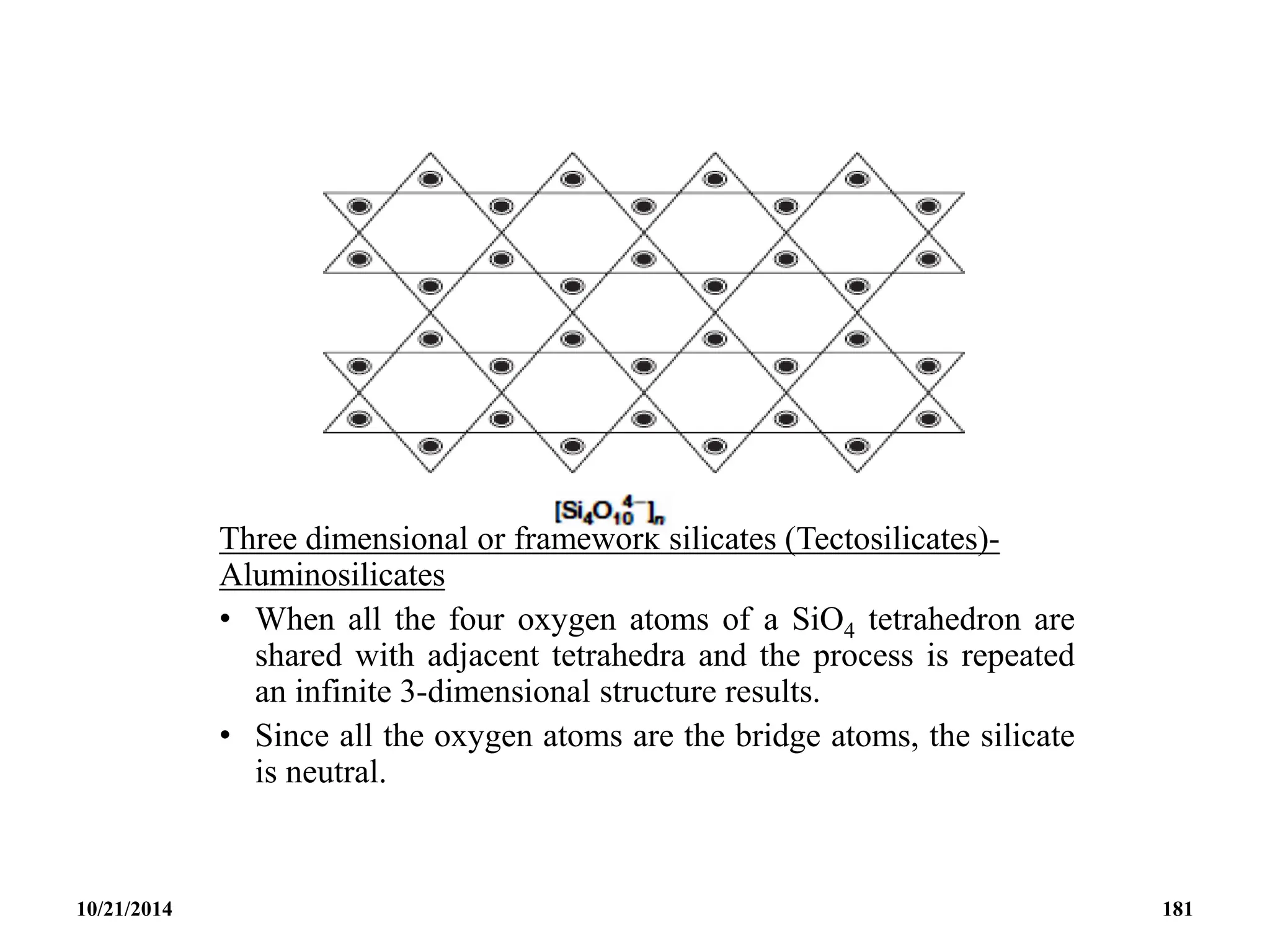
![STRUCTURAL CORRELATION
Very useful structural correlation between the Nido
and Arachno compounds is based on the observation
that clusters having the same number of skeletal
electrons are related by removal of such B-H groups
and the addition of the appropriate number of
electrons and H atoms.
This type of process relates the octahedral closo
[B6H6]2- anion to the square pyramidal nido-B5H9
borane which is in turn related to the butterfly-like
arachno-B4H10.
10/21/2014 22](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-22-2048.jpg)
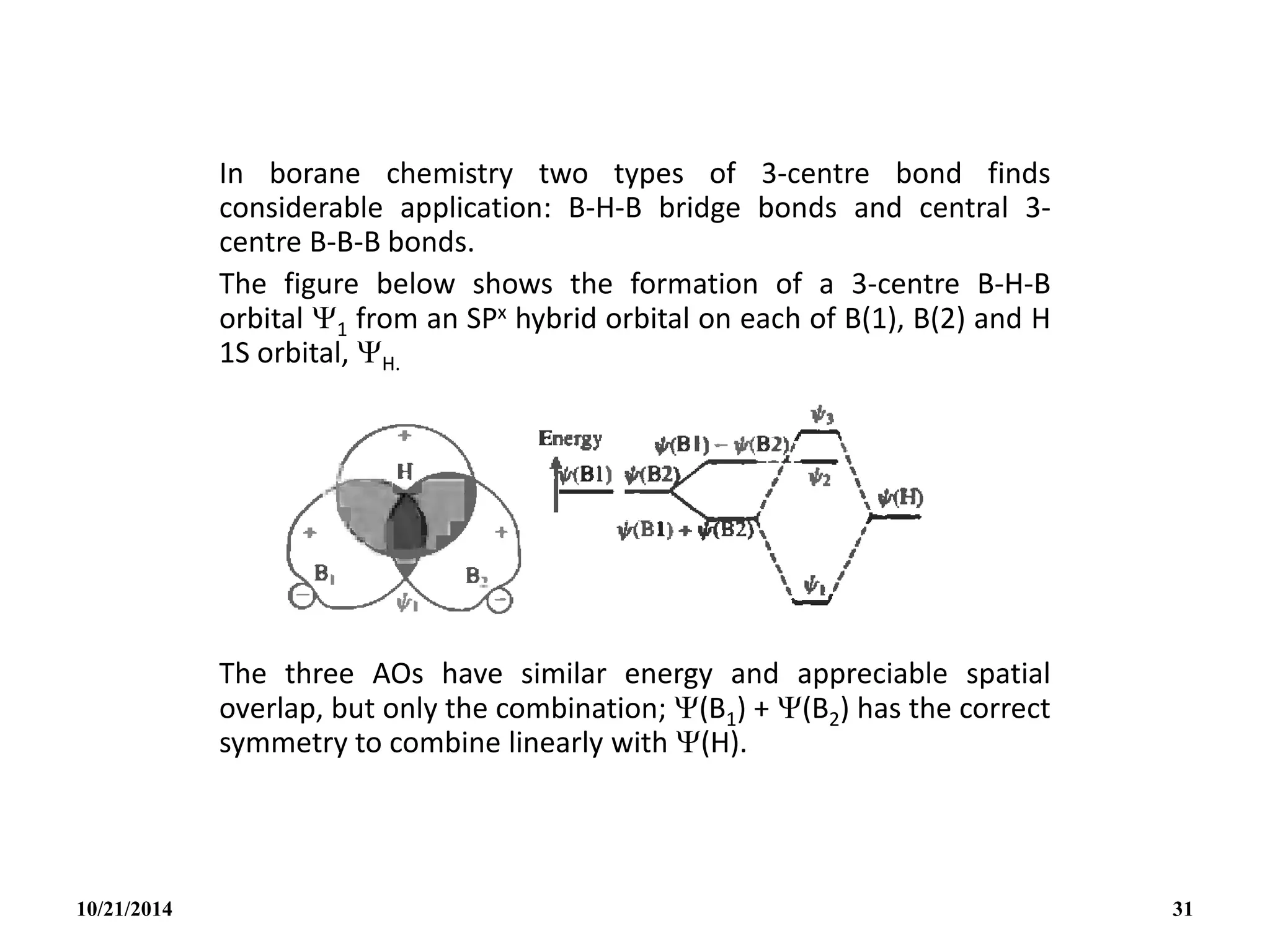
![The three normalized and orthogonal MOs have
the approximate form:
Bonding : 1 ½[(B1) + (B2)] + 1/2 (H)
Non-bonding (anti-bonding):
2 1/2 [(B1) - (B2)]
Anti-bonding : 3 ½[(B1) + (B2)] - 1/2 (H)
10/21/2014 32](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-32-2048.jpg)
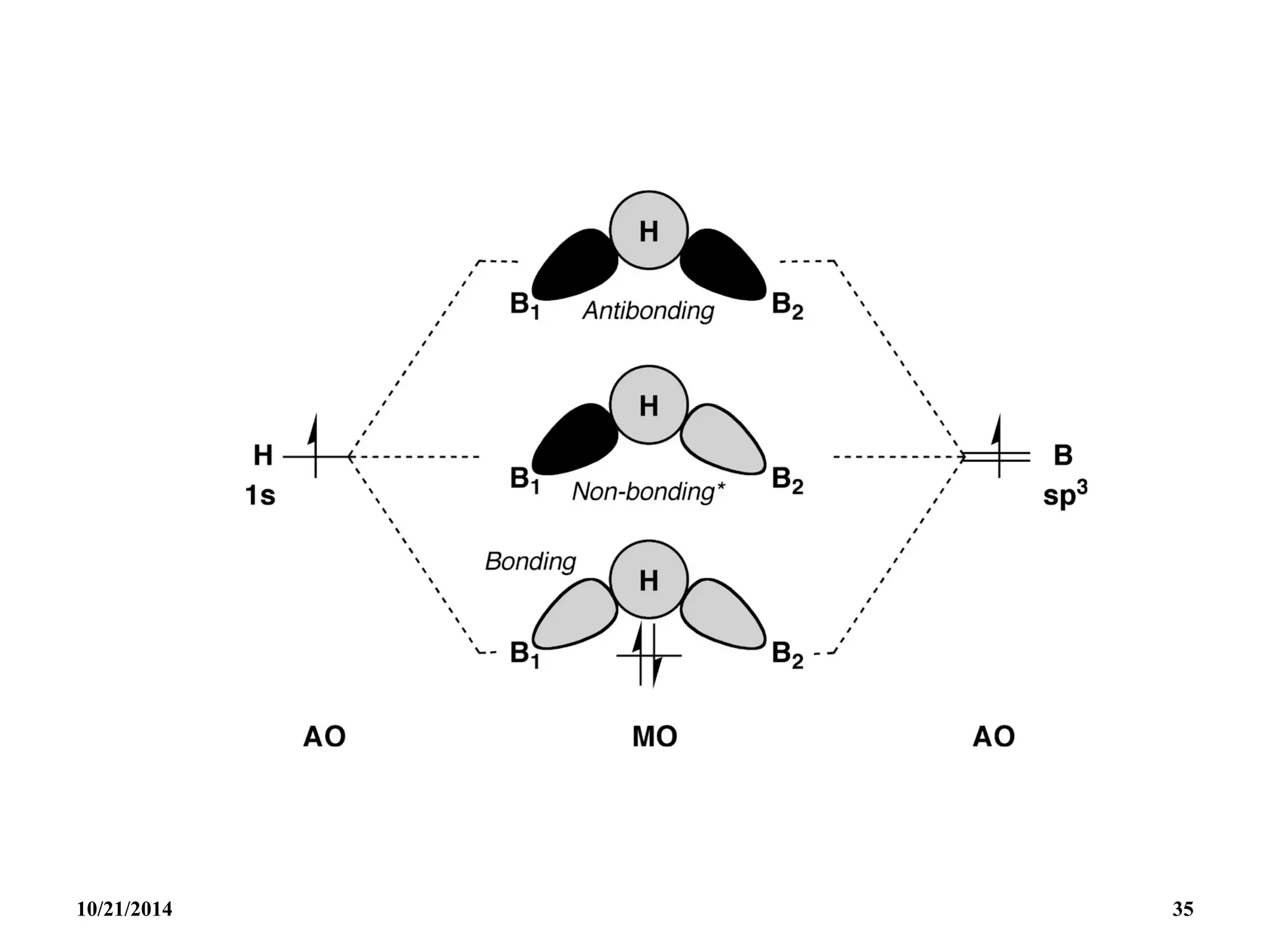
![Formation of a bonding central 3-centre bond 1 and schematic
representation of the relative energies of the 3 molecular orbitals
1, 2 and 3.
The approximate analytic forms of these MOs are:
Bonding : 1 [(B1) + (B2) + (B3)]/3
Anti-bonding : 2 [(B1) - (B2)]/2
Anti-bonding : 3 [(B1) + (B2) - 2(B3)]/6
For closo and for larger open cluster boranes it becomes
increasingly difficult to write a simple satisfactory localized
orbital structure, and full MO treatment is required.
10/21/2014 33](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-33-2048.jpg)

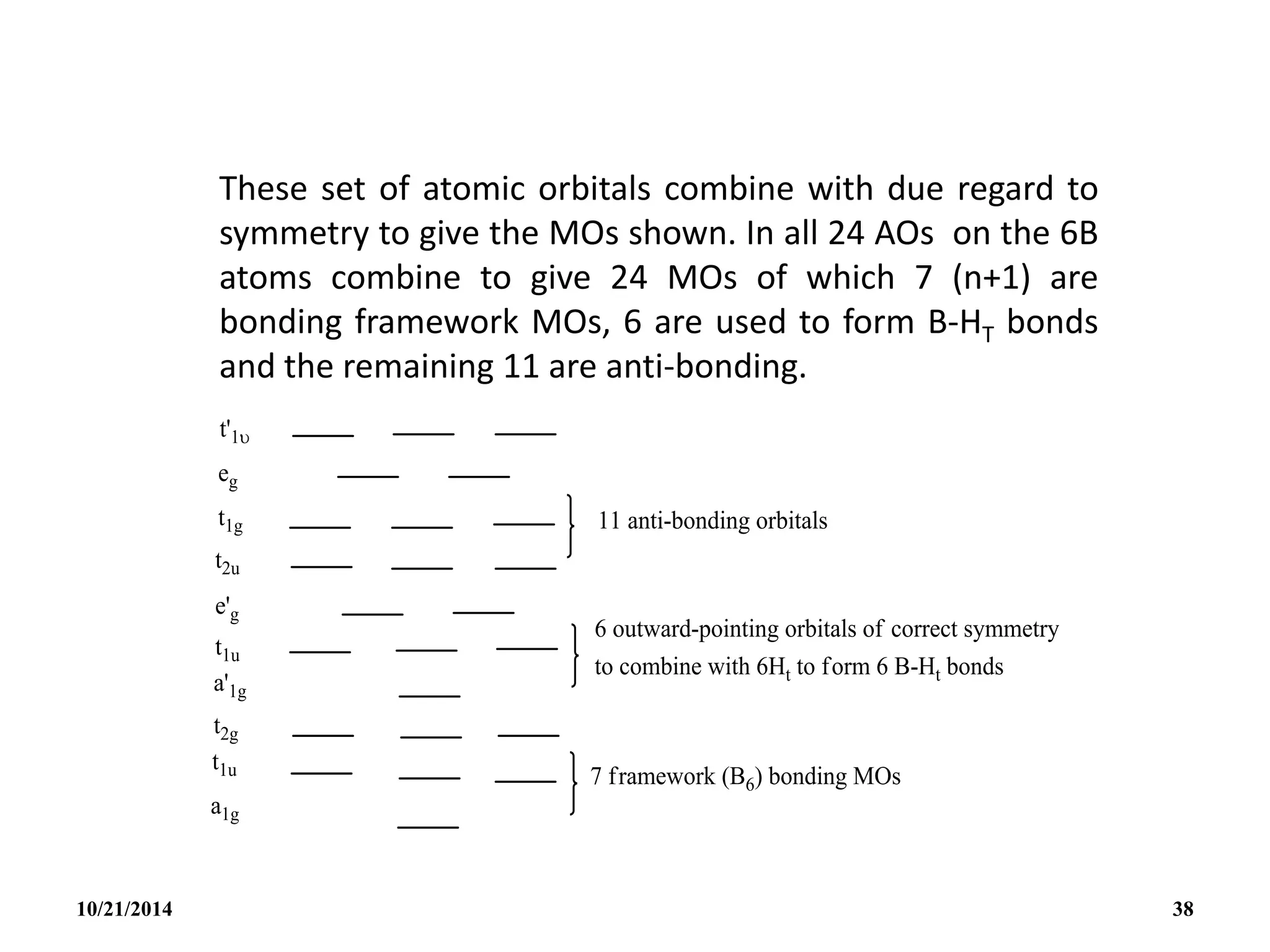


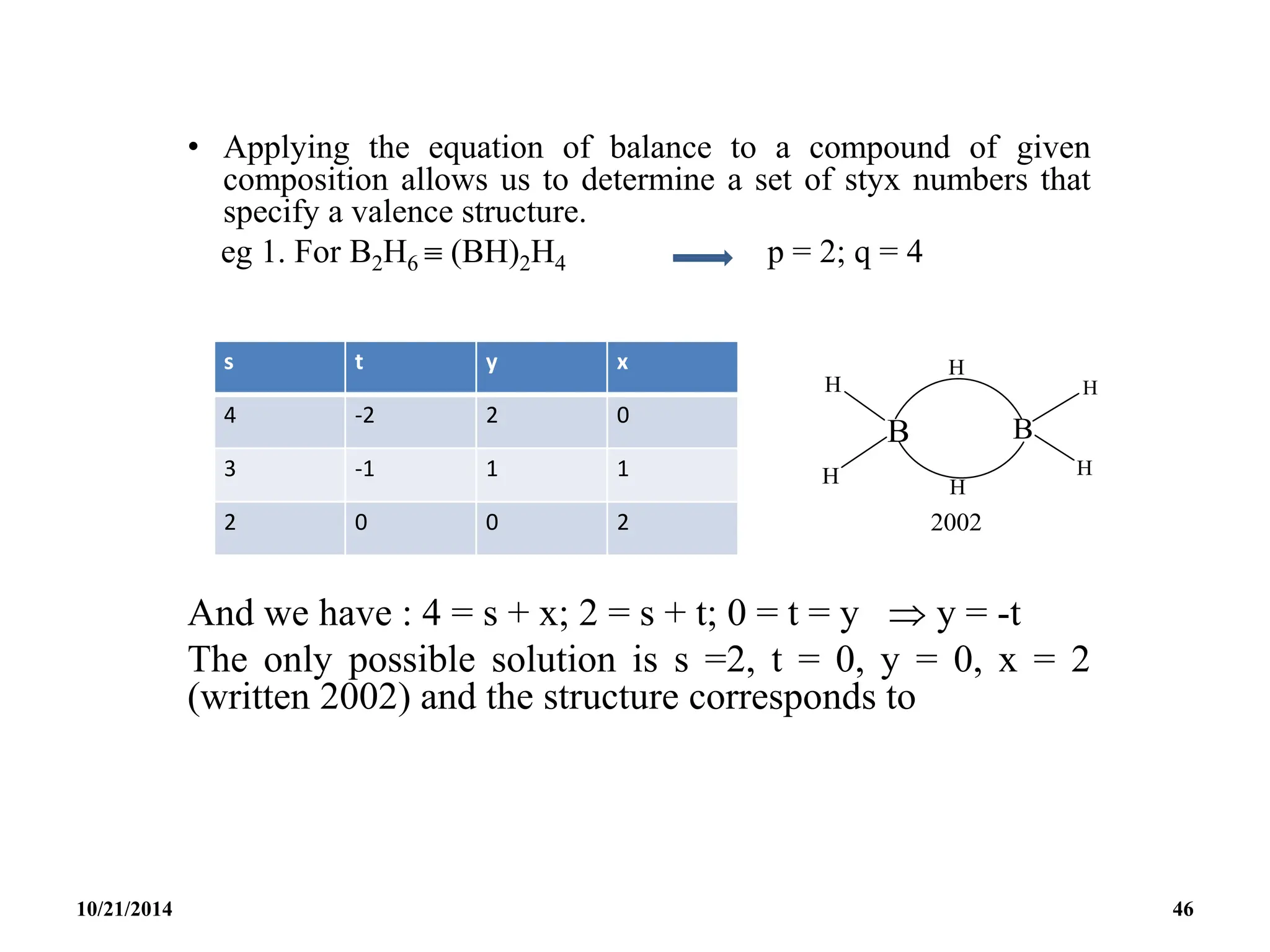
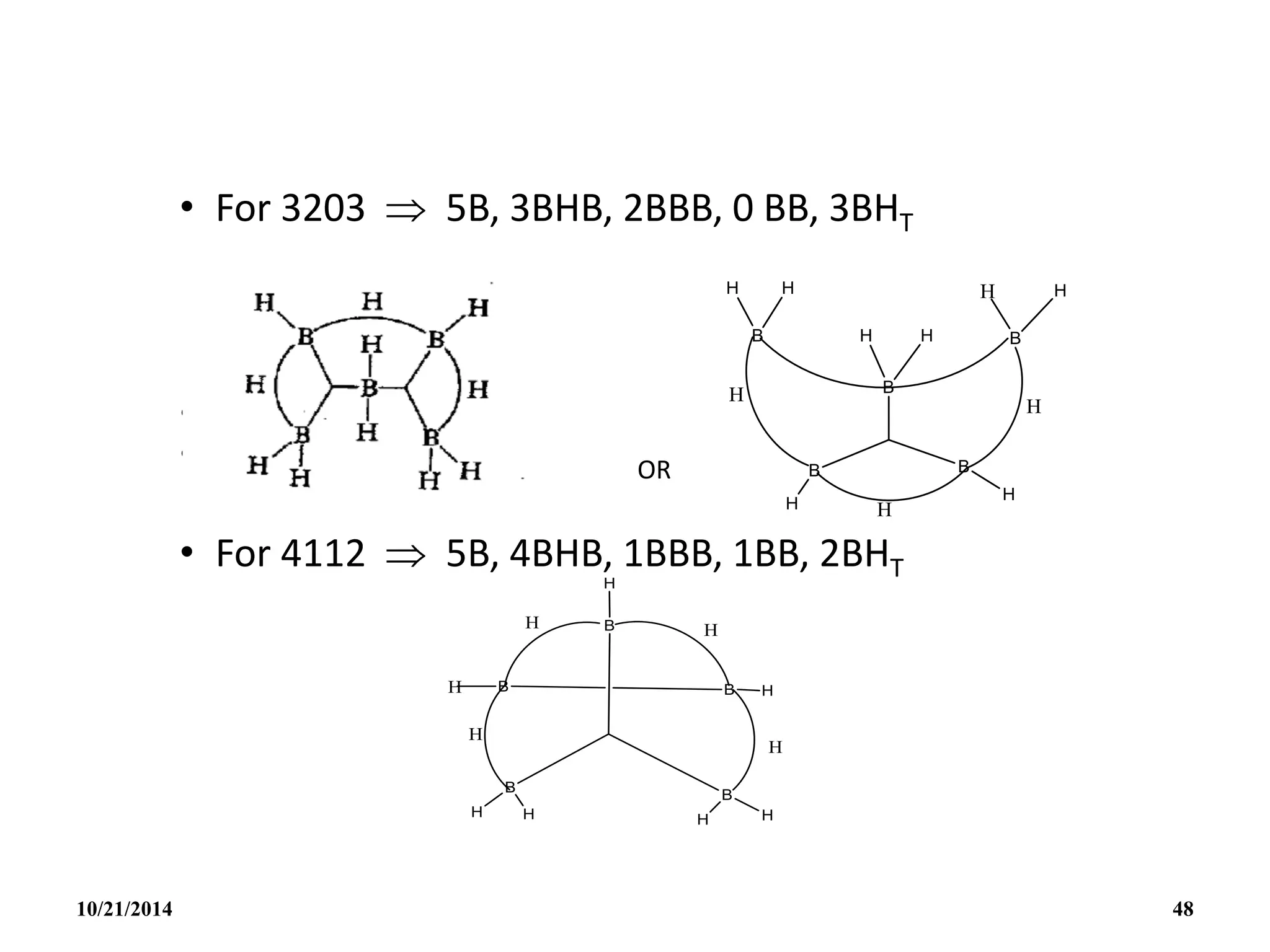


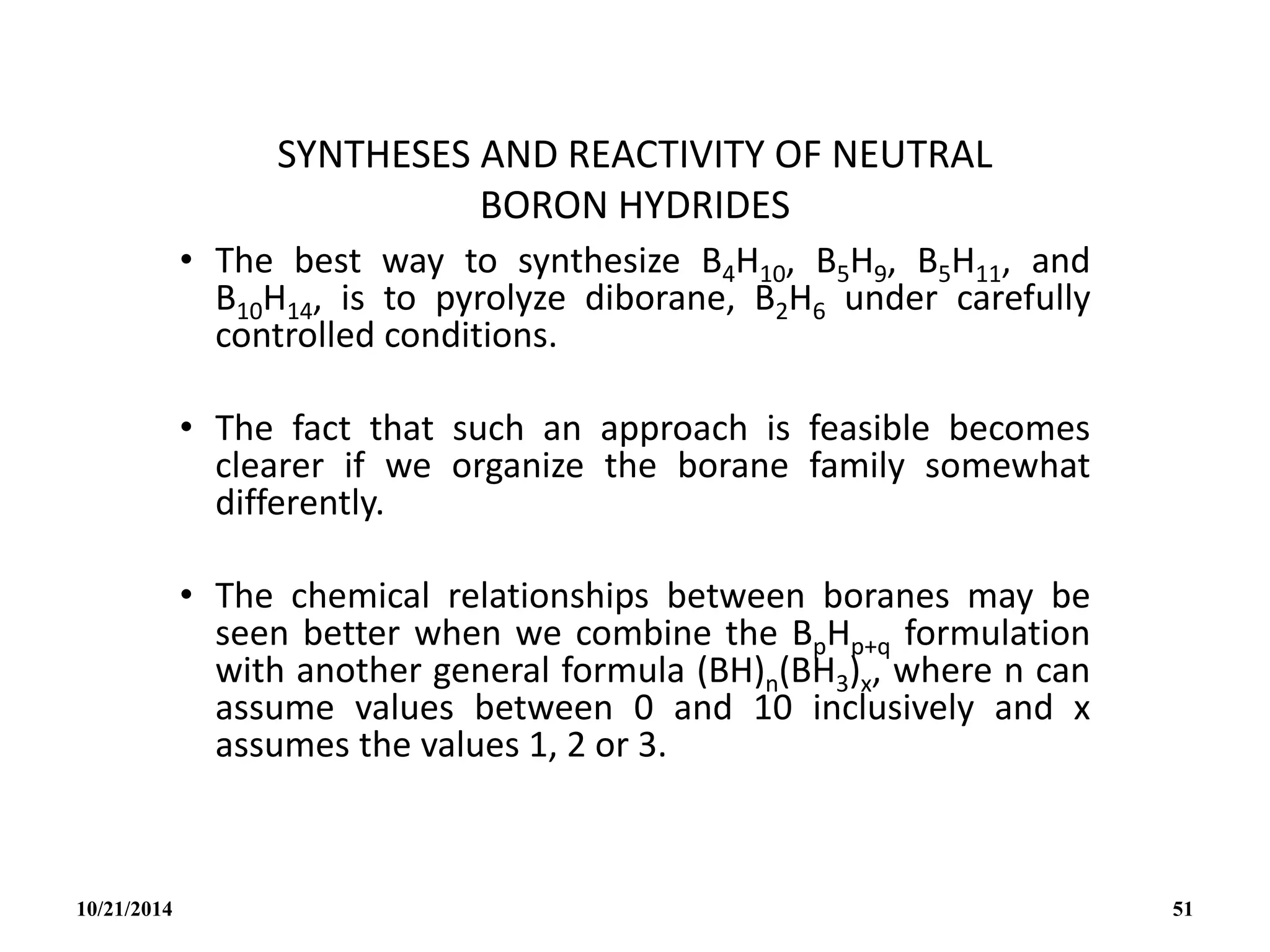
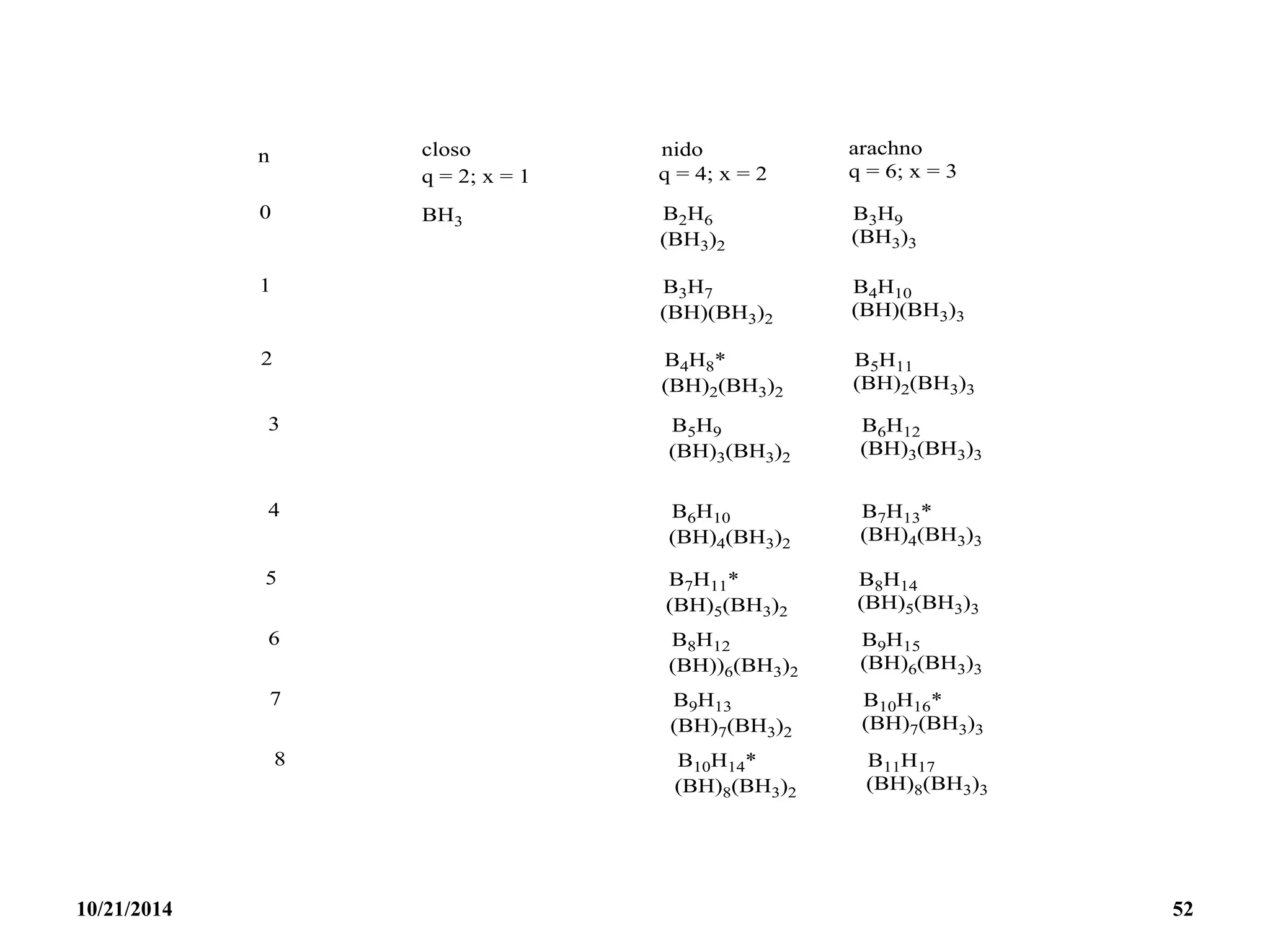

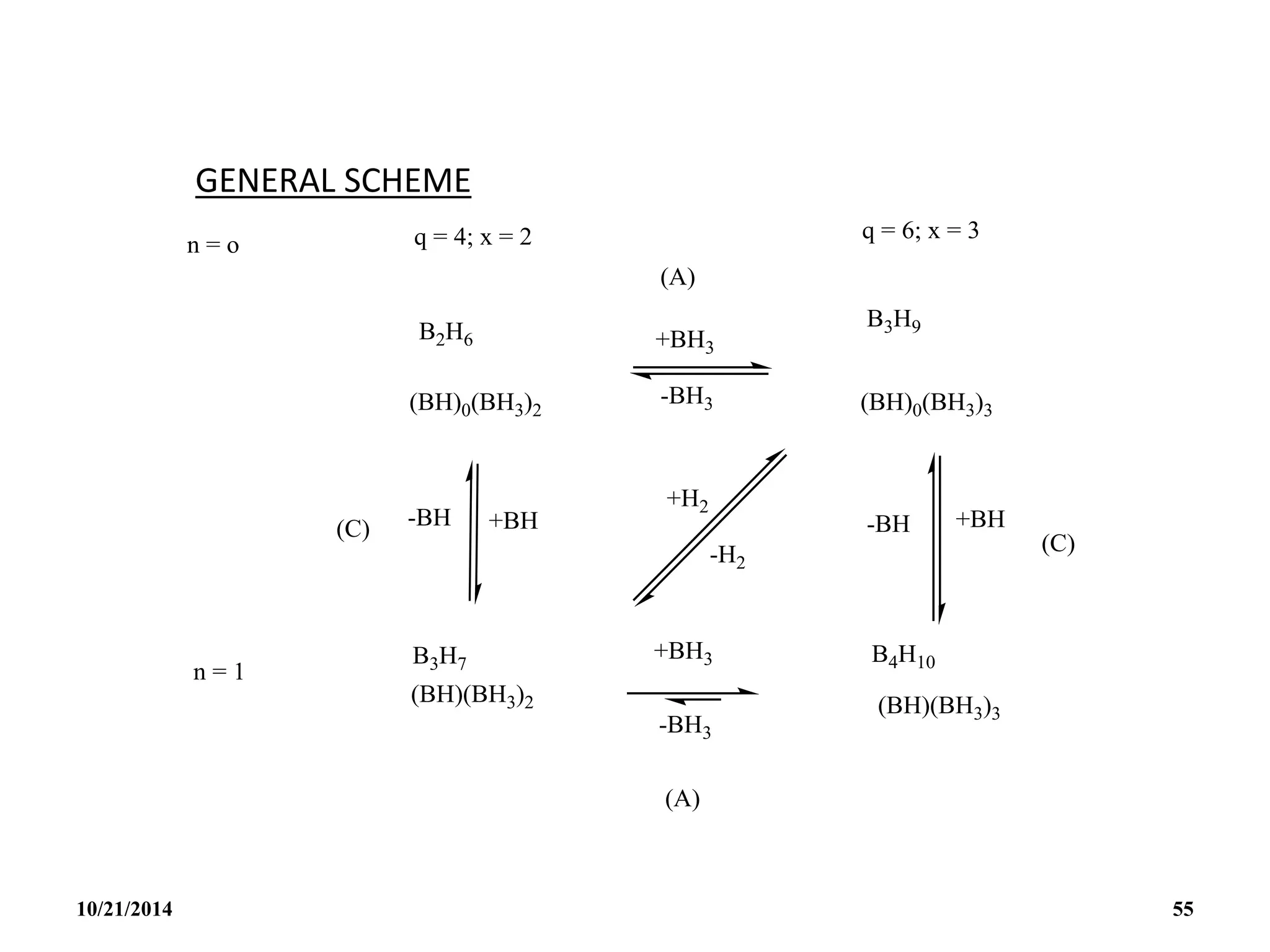
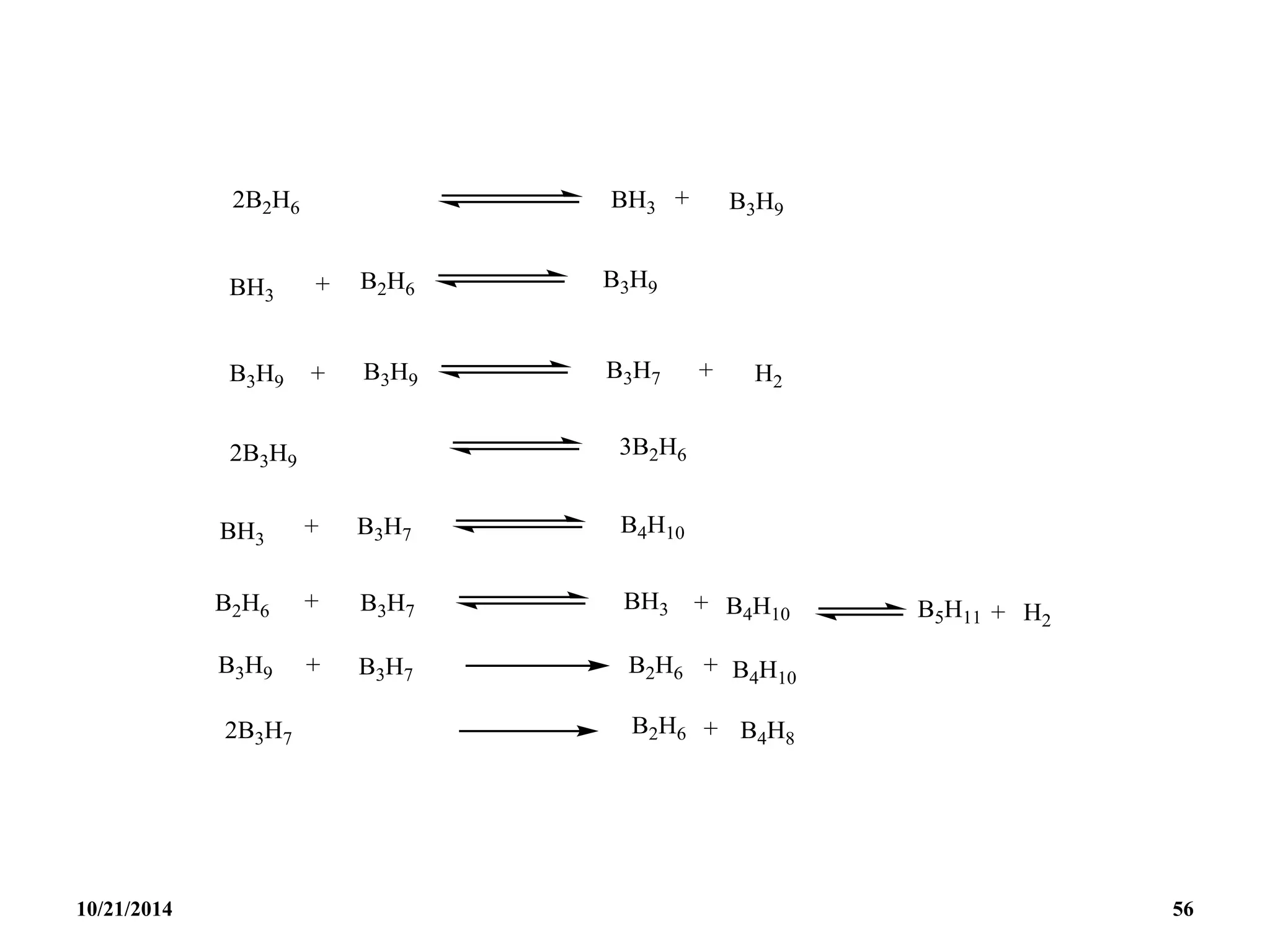
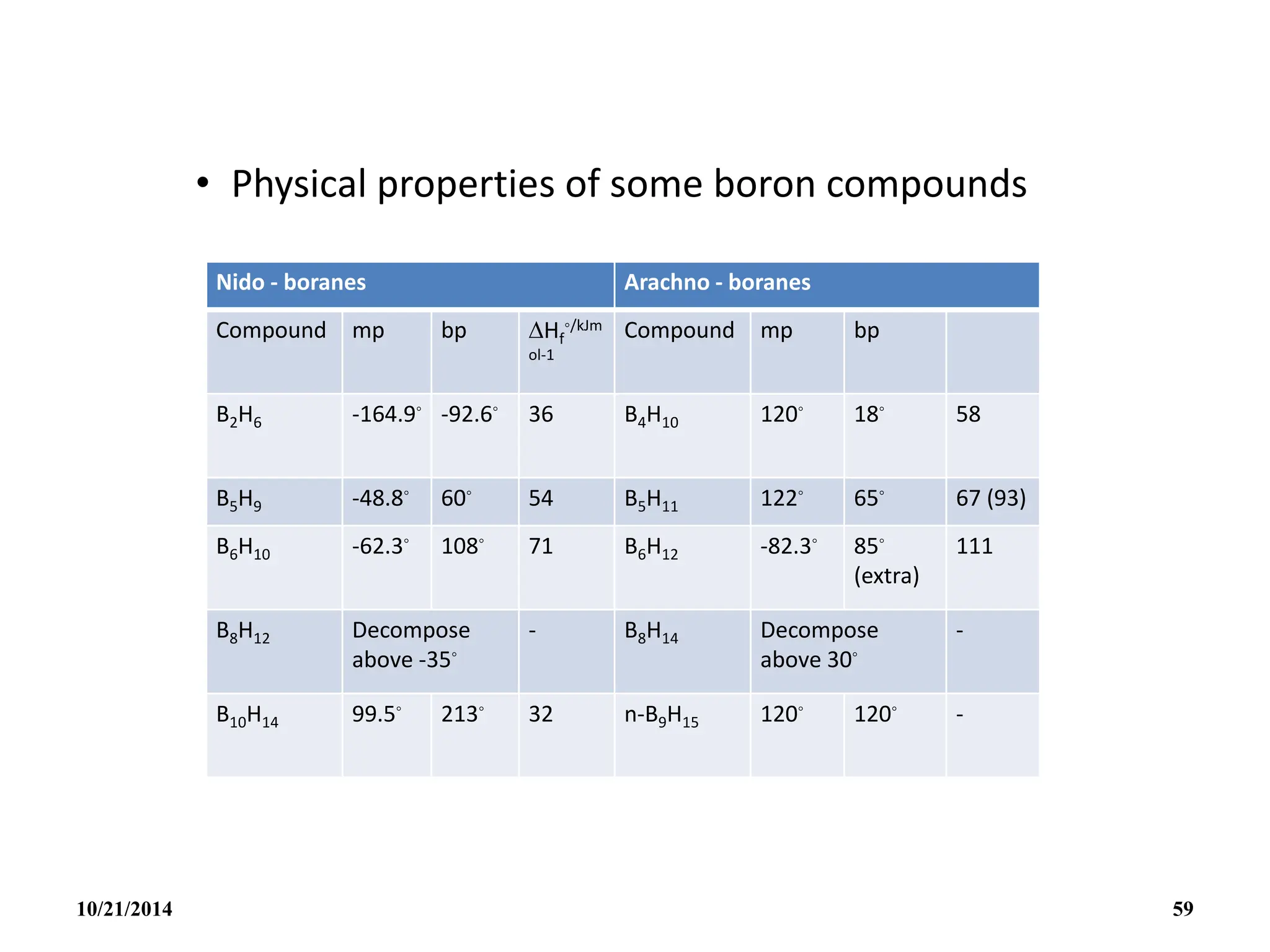
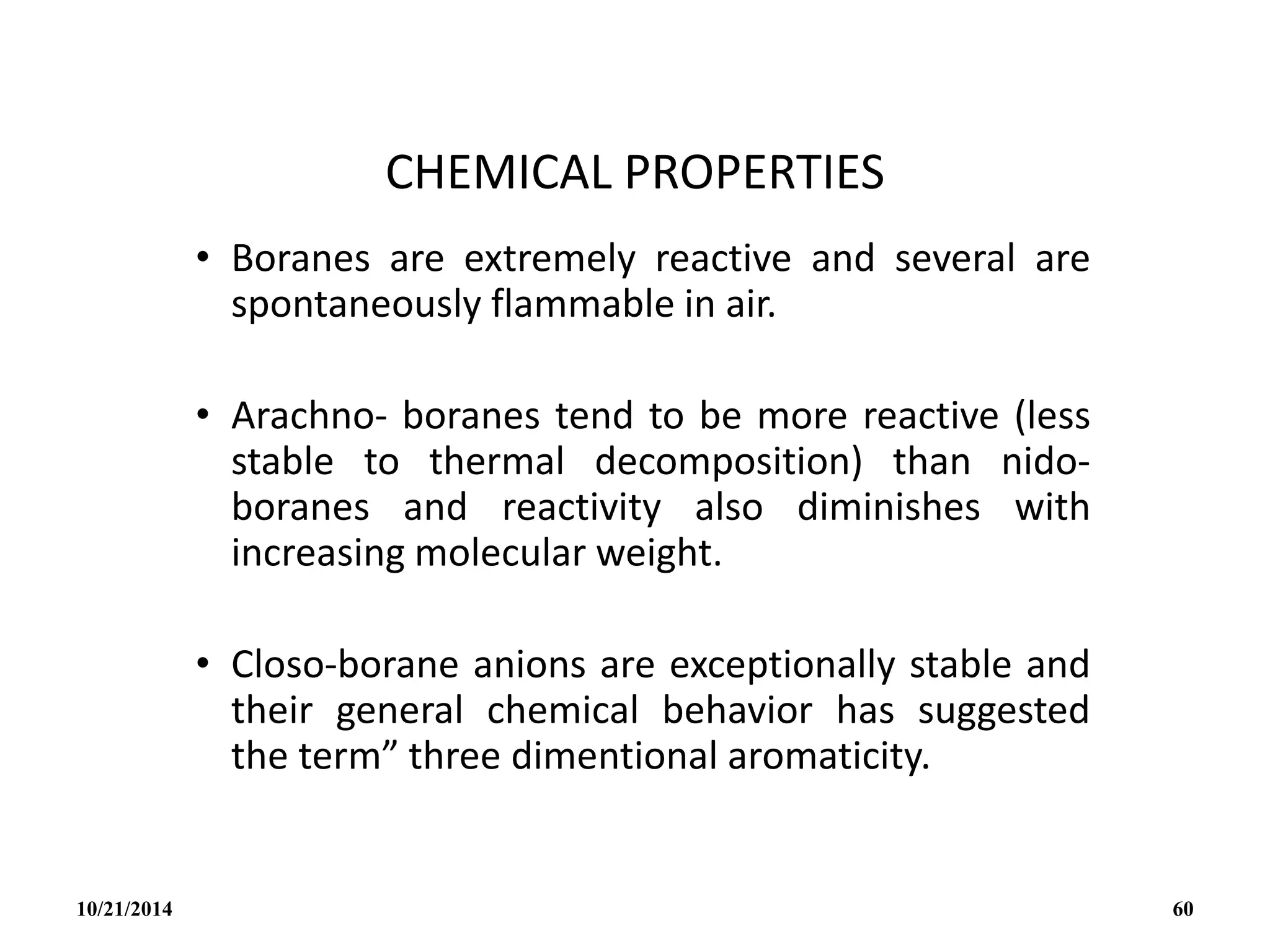
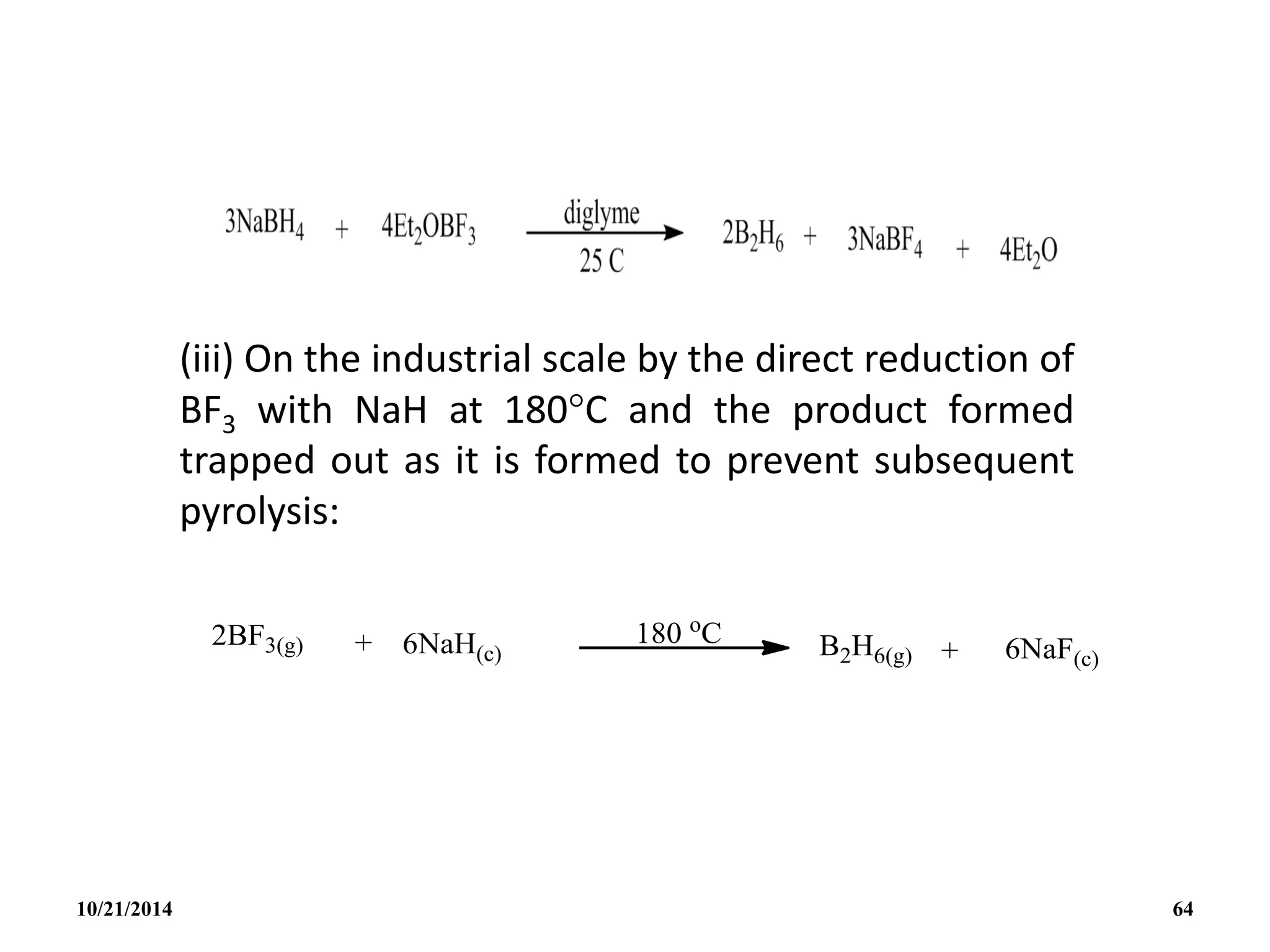
![PREPARATION
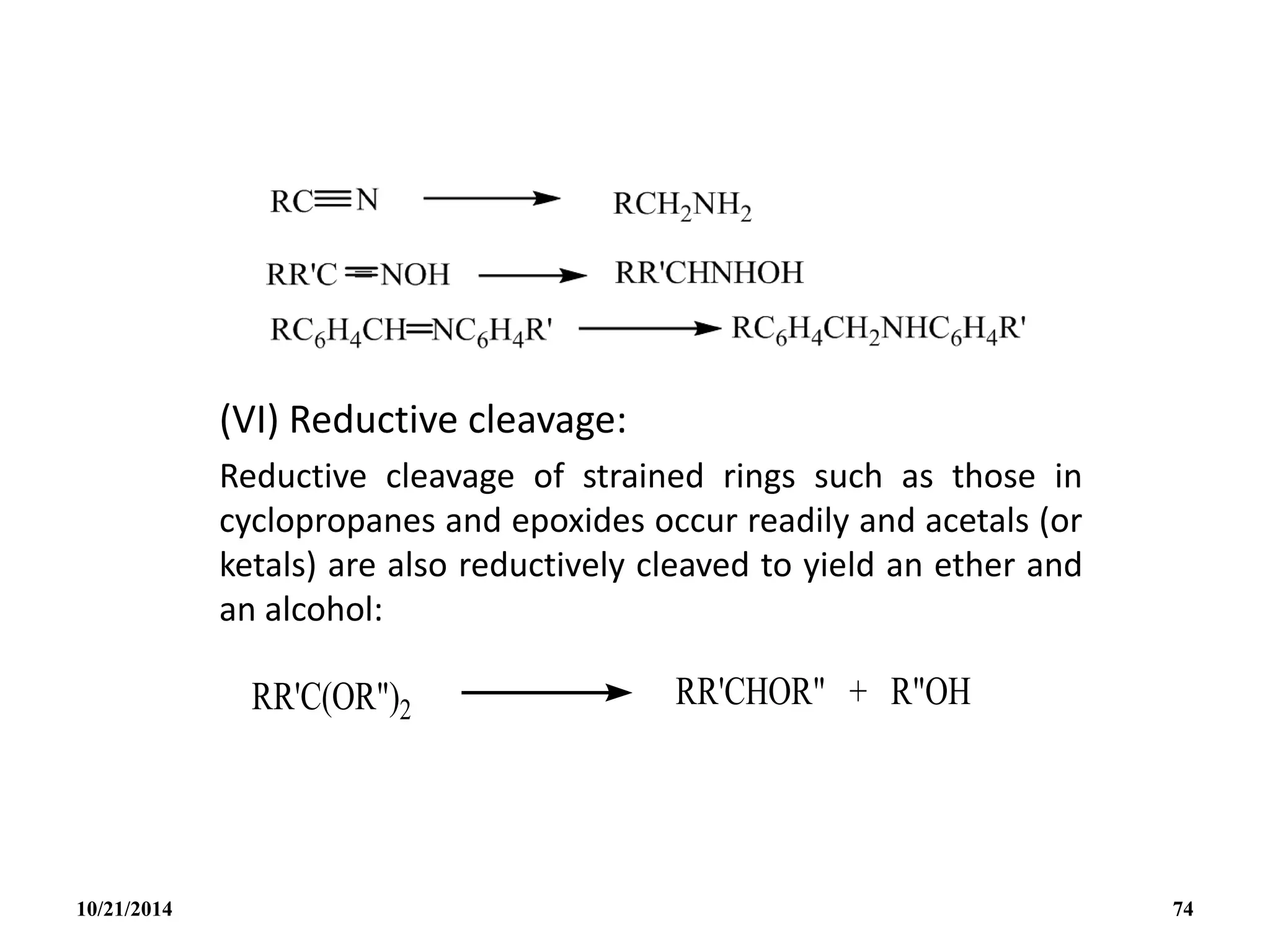
(i) B2H6 gas is most conveniently prepared in small
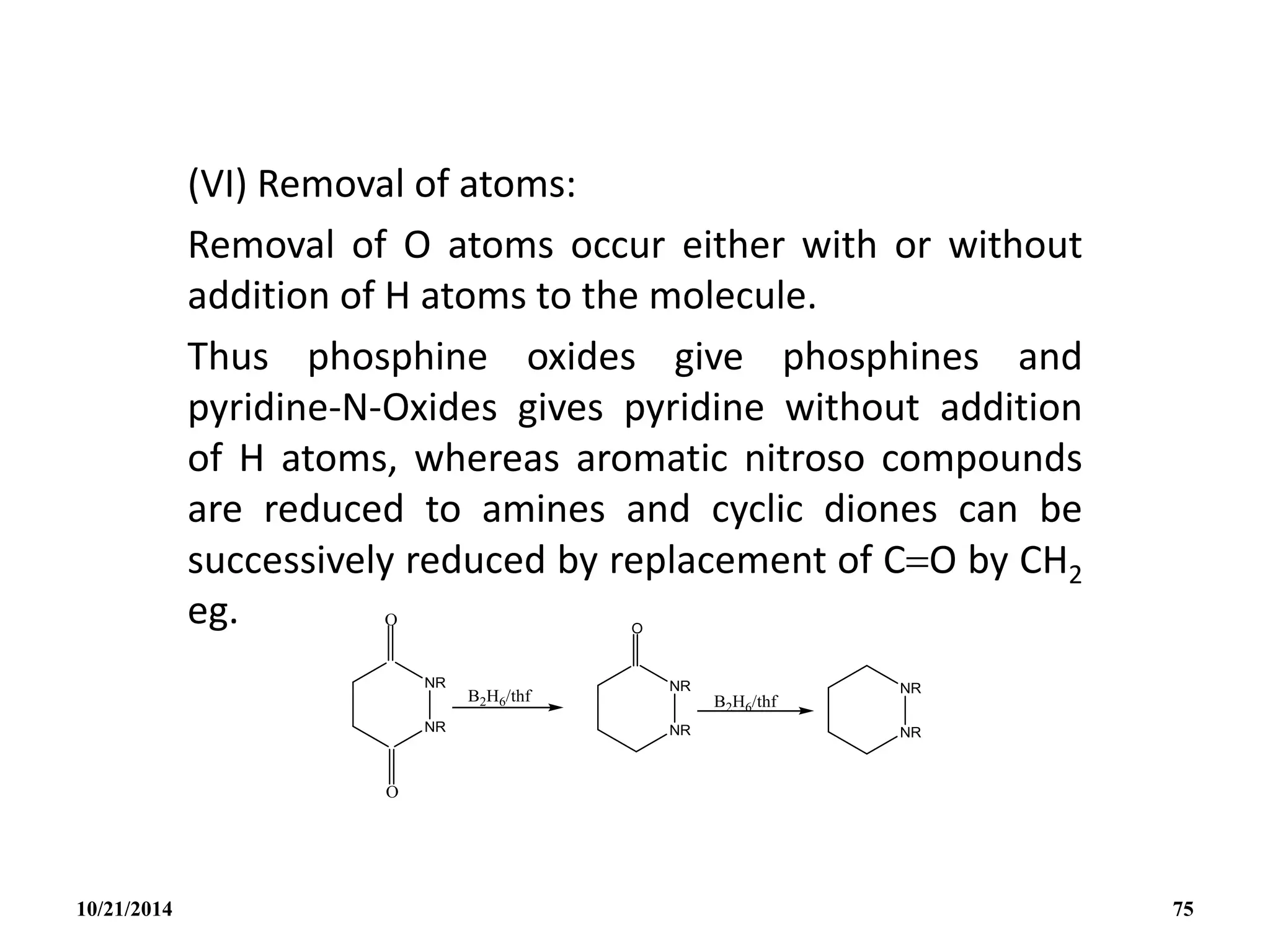
quantities by the reaction of I2 on NaBH4 in
diglyme[(MeOCH2CH2)2O] or by the reaction of a solid
tetrahydroborate with an anhydrous acid:
(ii) When B2H6 is used as reaction intermediate without the
need for isolation or purification the best procedure is to
add Et2OBF3 to NaBH4 in a polyether such as diglyme.
10/21/2014 63](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-63-2048.jpg)
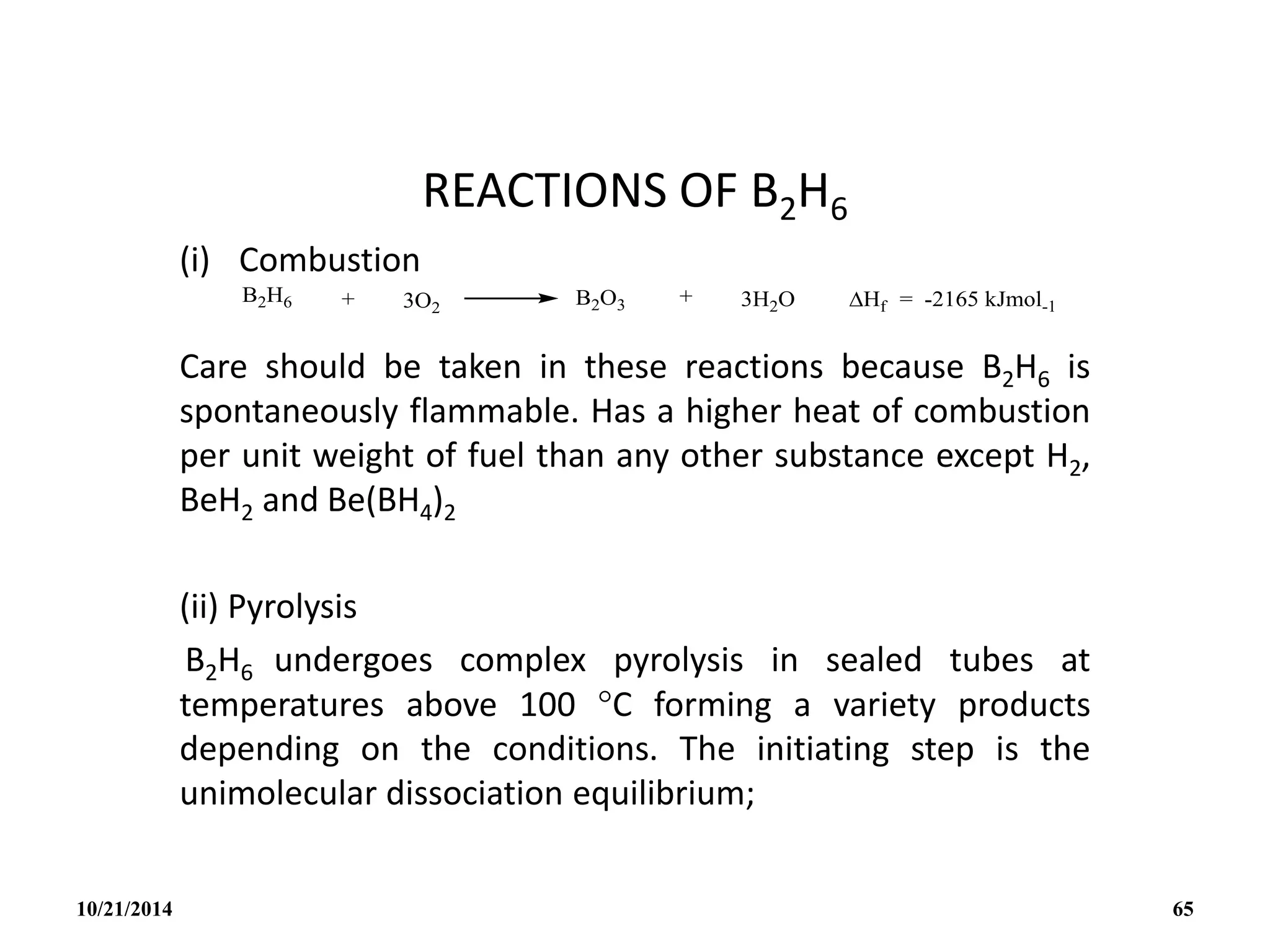
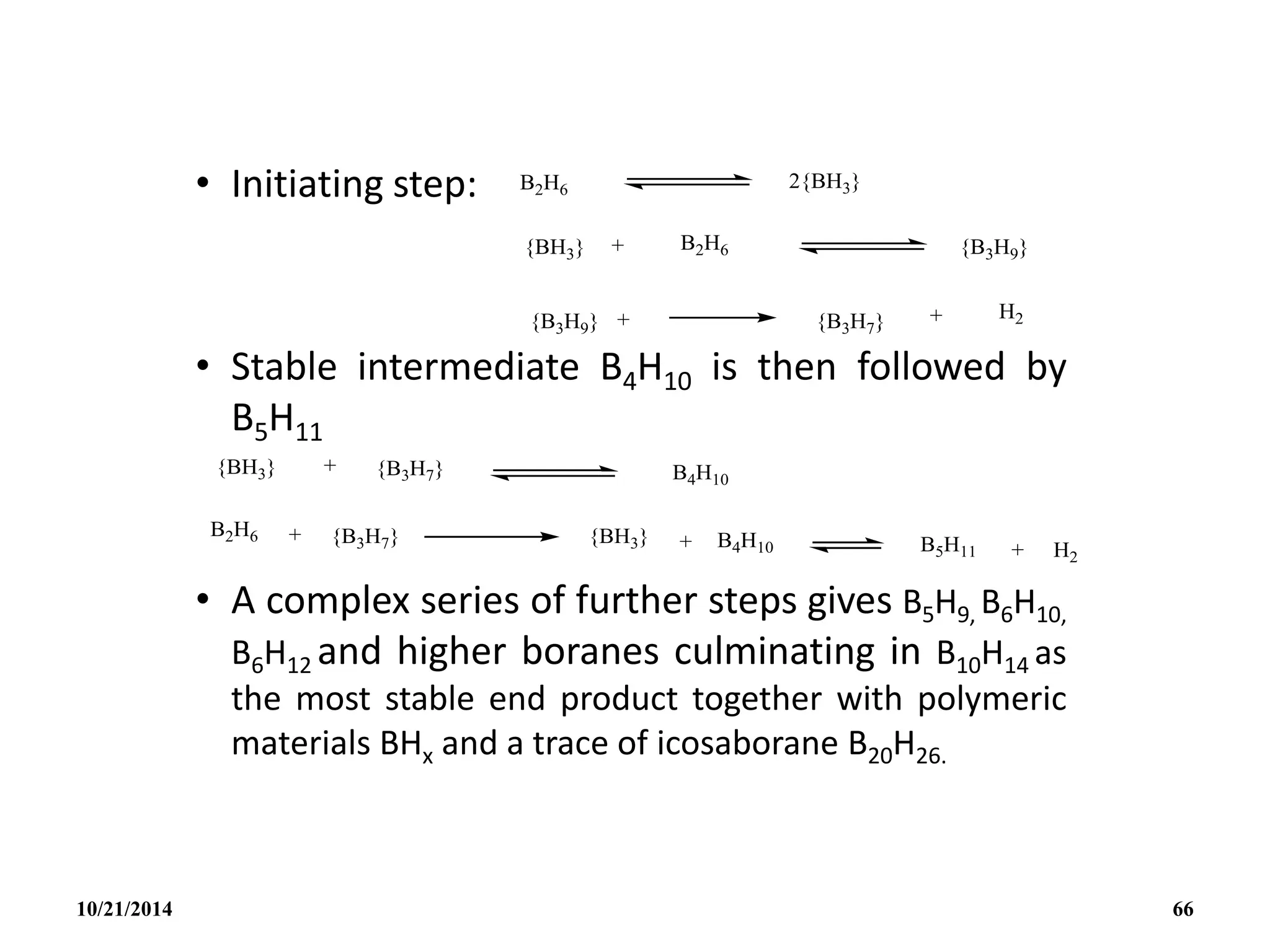
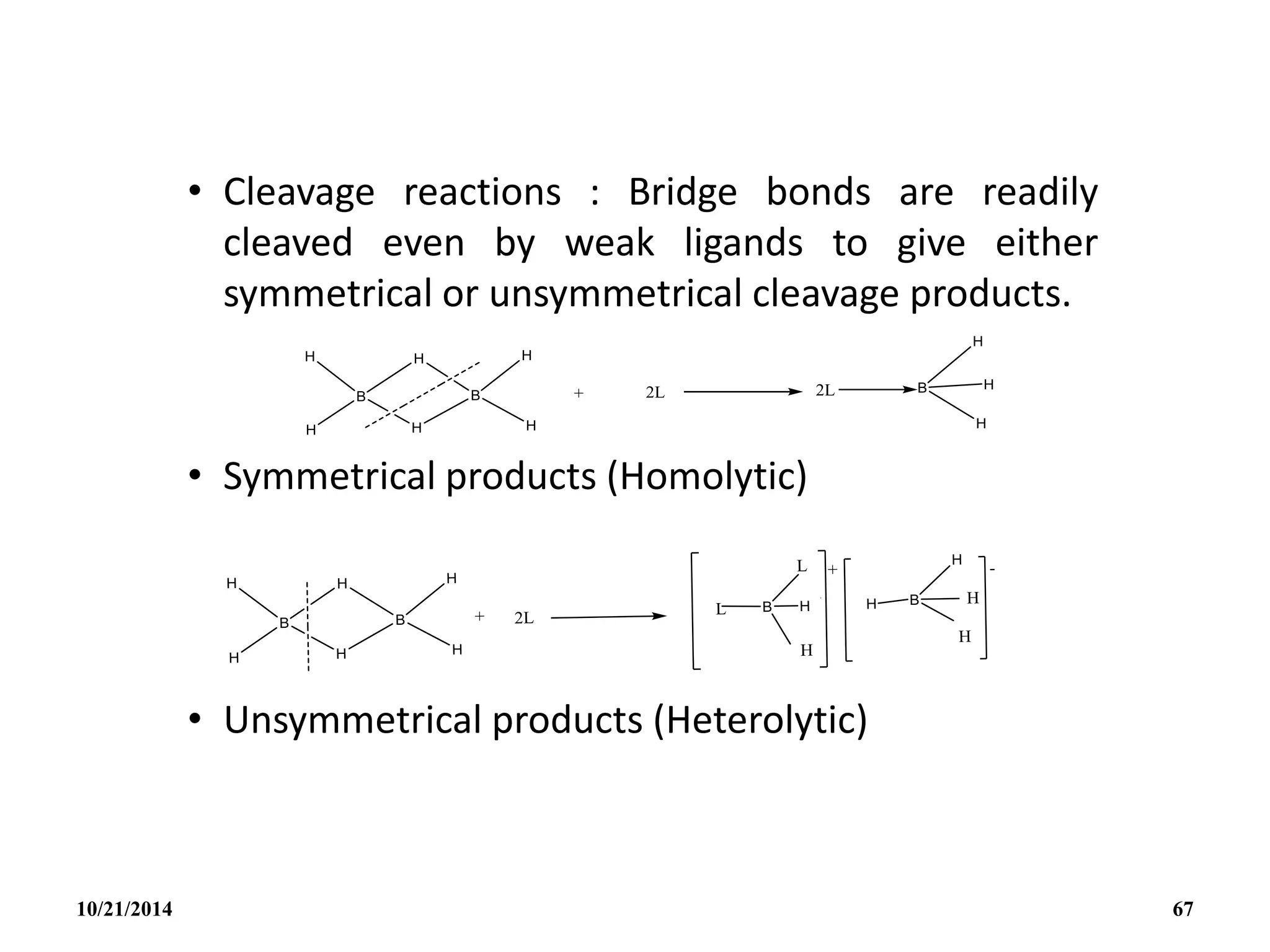
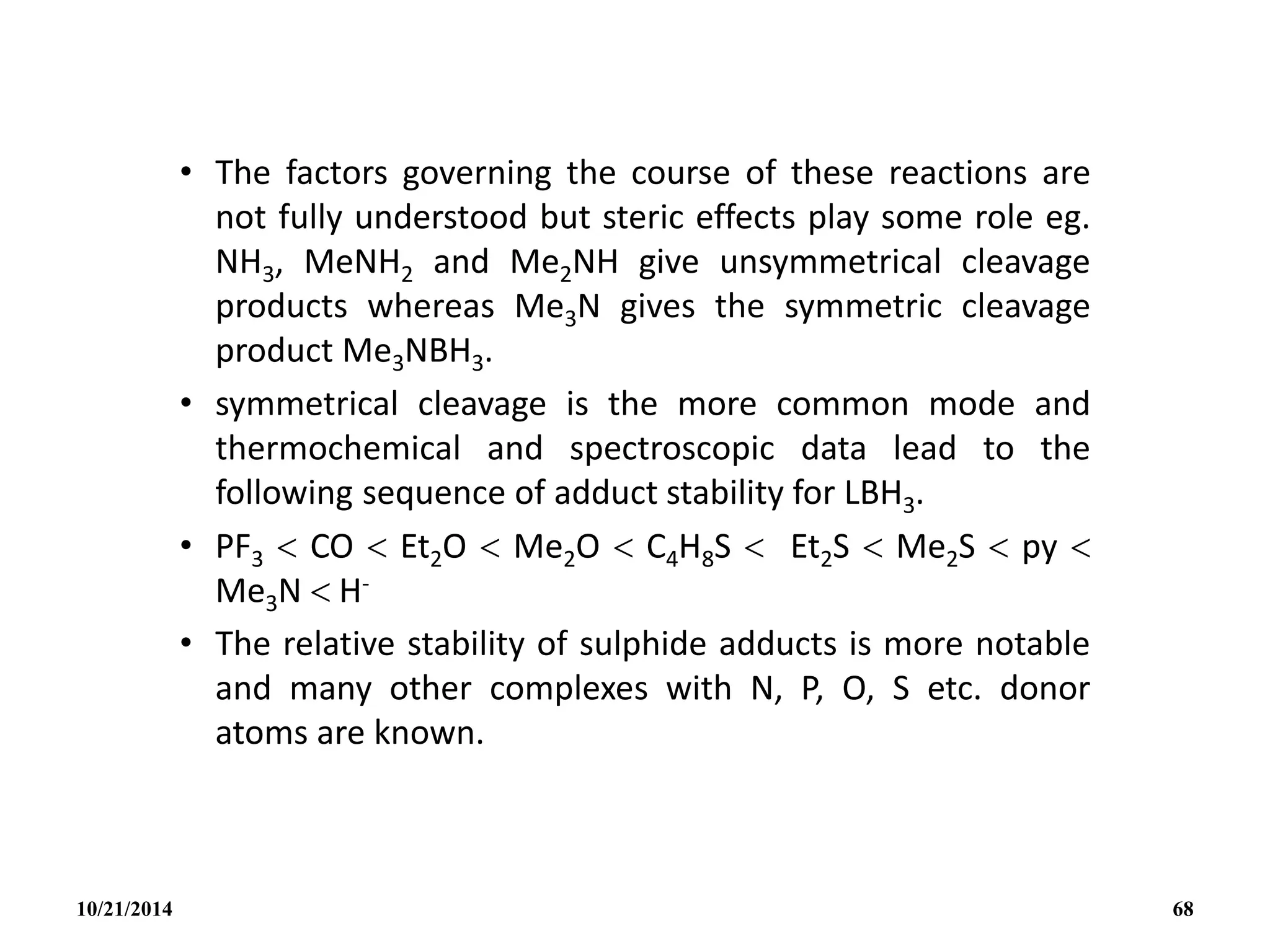
![• The H- is a special case since it gives the
symmetrical tetrahedral ion BH4
- isoelectronic
with CH4.
• The BH4
- ion itself provide a rare example of a
ligand that can be unidentate, bidentate or
tridentate (eg. [cu1( 1- BH4)(PMePh2)3], [Cu1(
2 – BH4)(PPh3)2]; [ZrIV(3 – BH4)4]).
• In addition to pyrolysis and cleavage
reactions, B2H6 undergoes a wide range of
substitution, redistribution and solvolytic
reactions:
10/21/2014 69](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-69-2048.jpg)
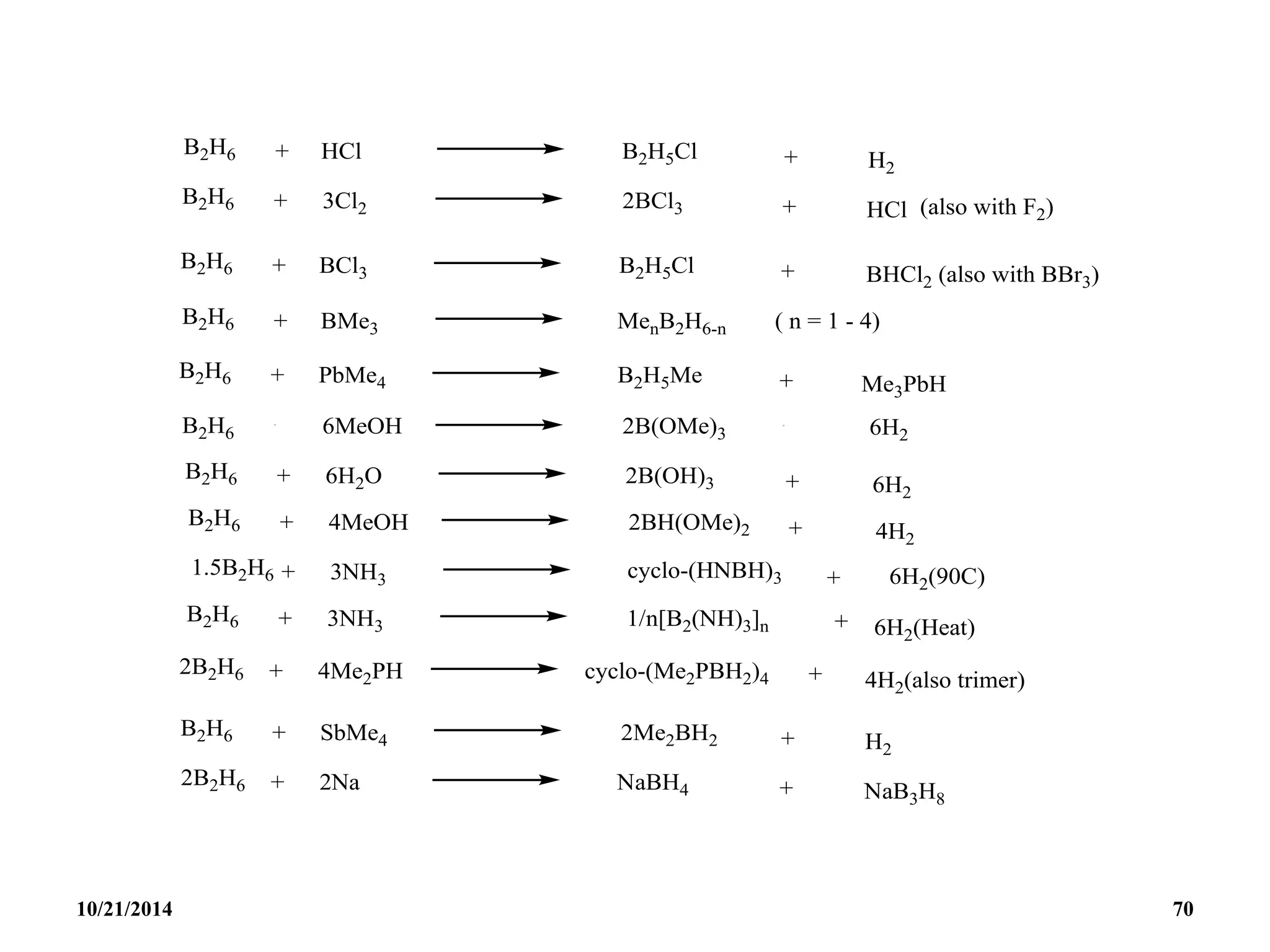
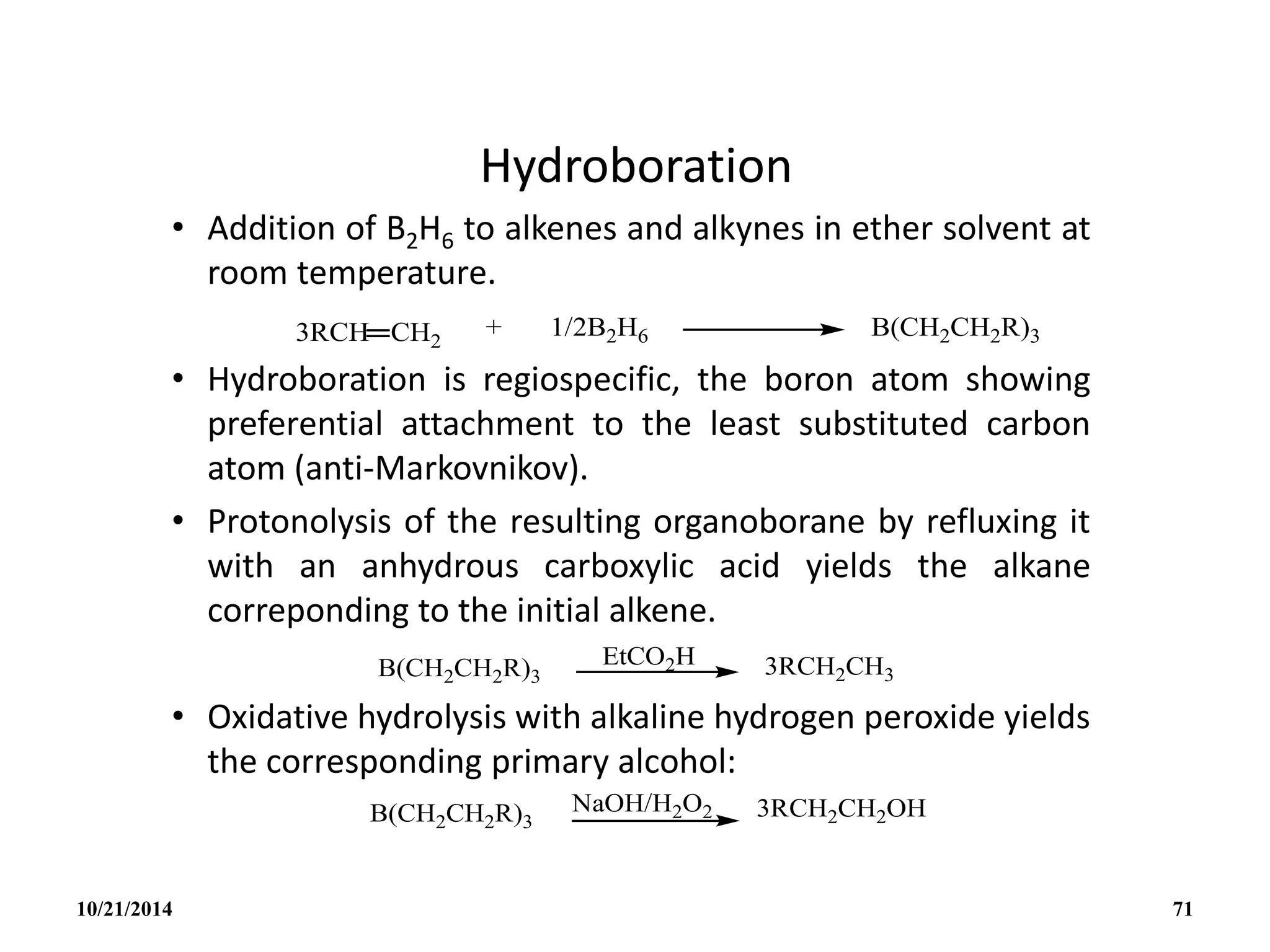
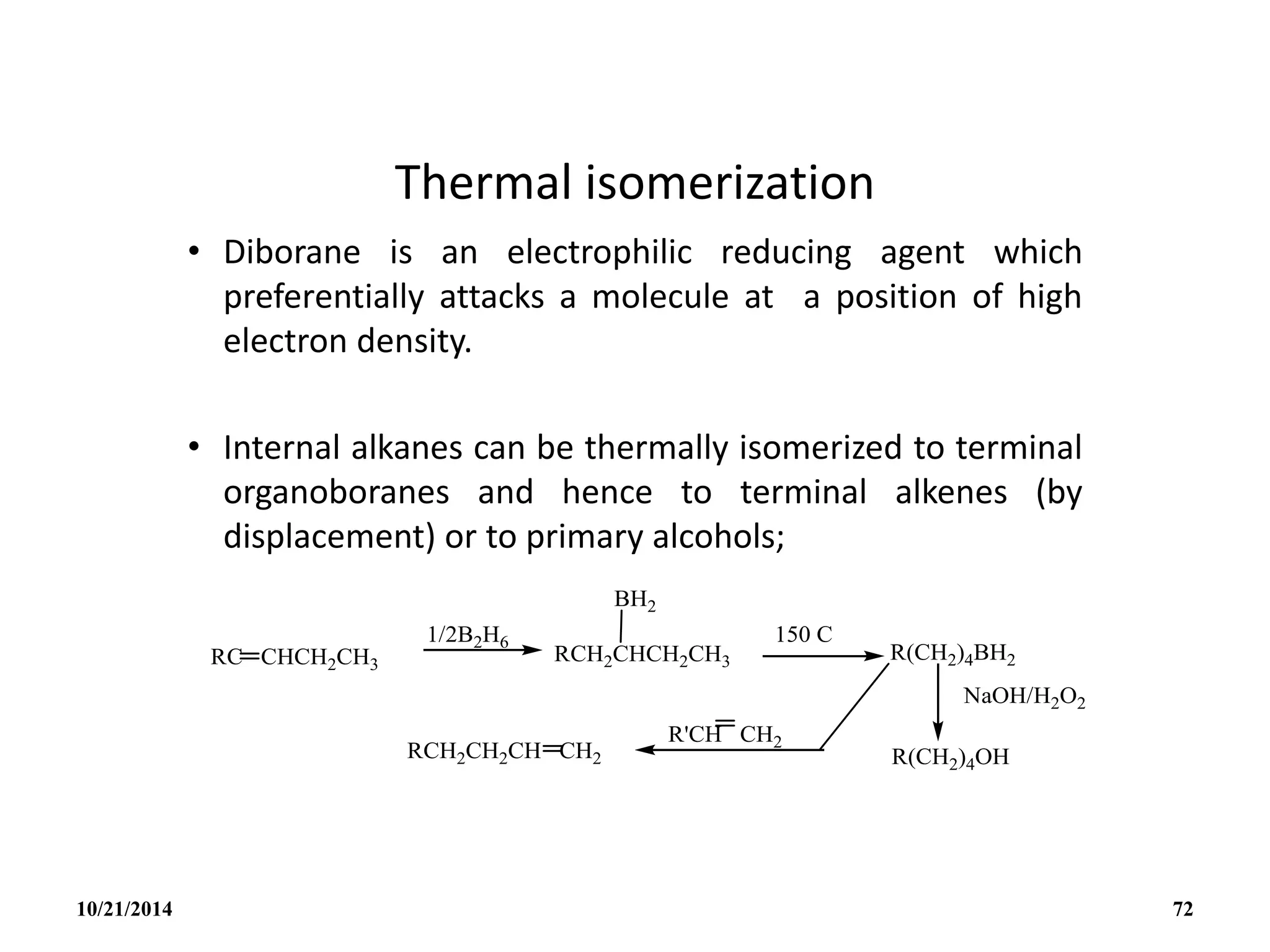
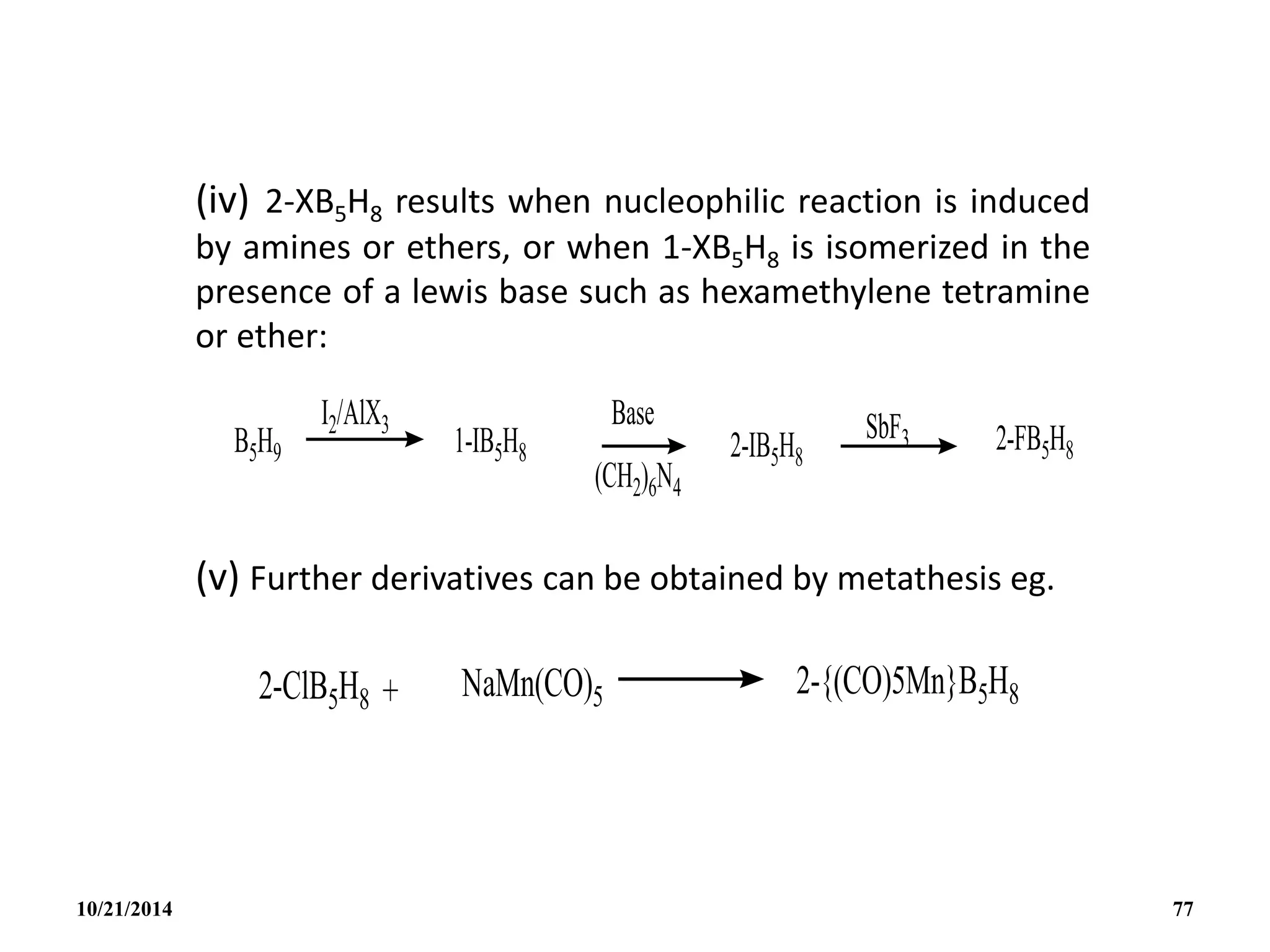
![REACTIONS
(i) Lewis bases
B5H9 reacts with lewis bases (electron pair donors) to form
adducts eg with PMe3 to give [B5H9(PMe3)2].
(ii) Weak Bronsted acid:
B5H9 acts as a weak acid. The acidity increases with
increasing size of the borane cluster and arachno-boranes
are more acidic than nido-boranes.
Nido : B5H9 B6H10 B10H14 B16H20 B18H22
Arachno : B4H10 B5H11 B6H12 and B4H10 B6H10
10/21/2014 78](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-78-2048.jpg)
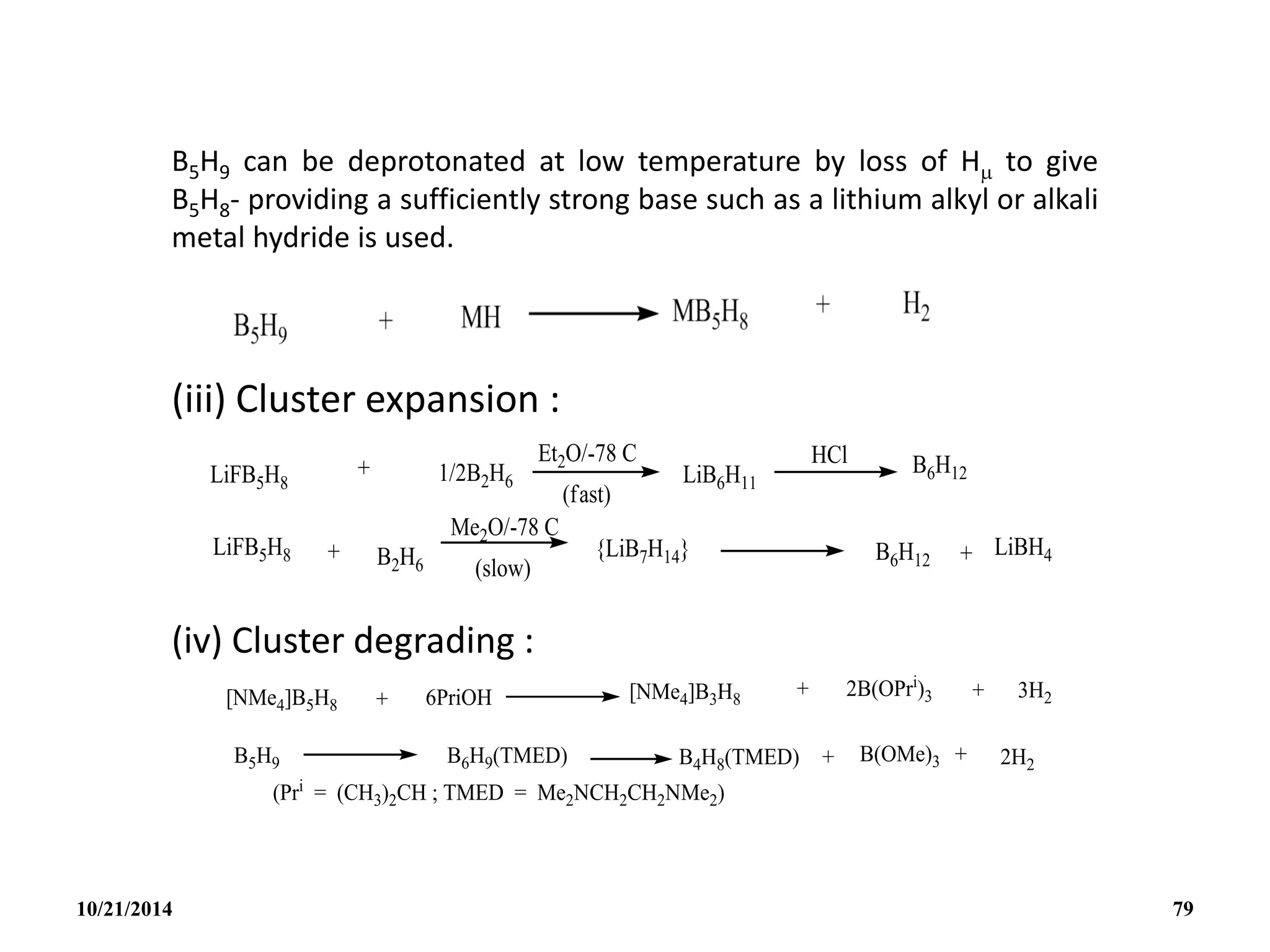
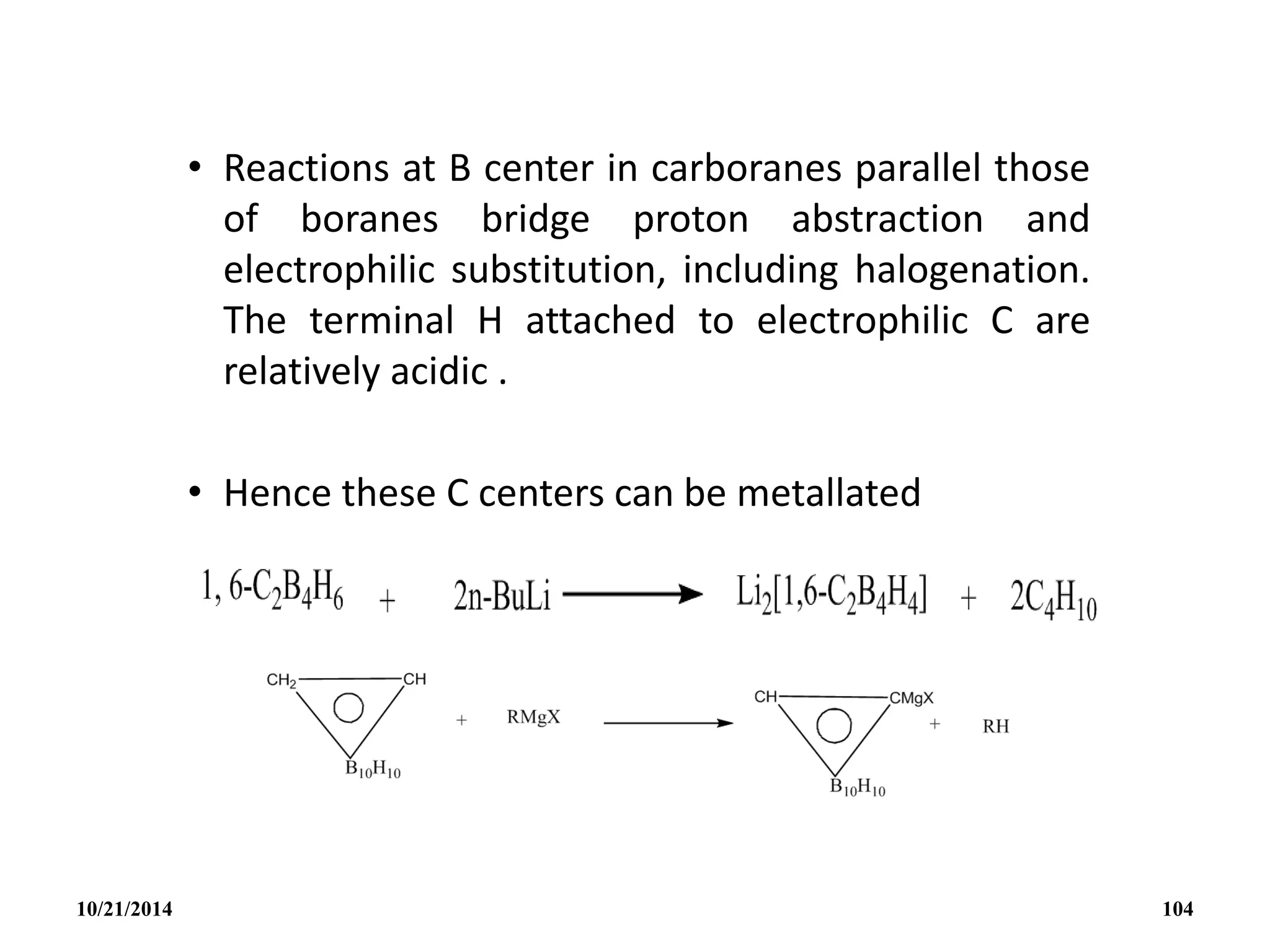
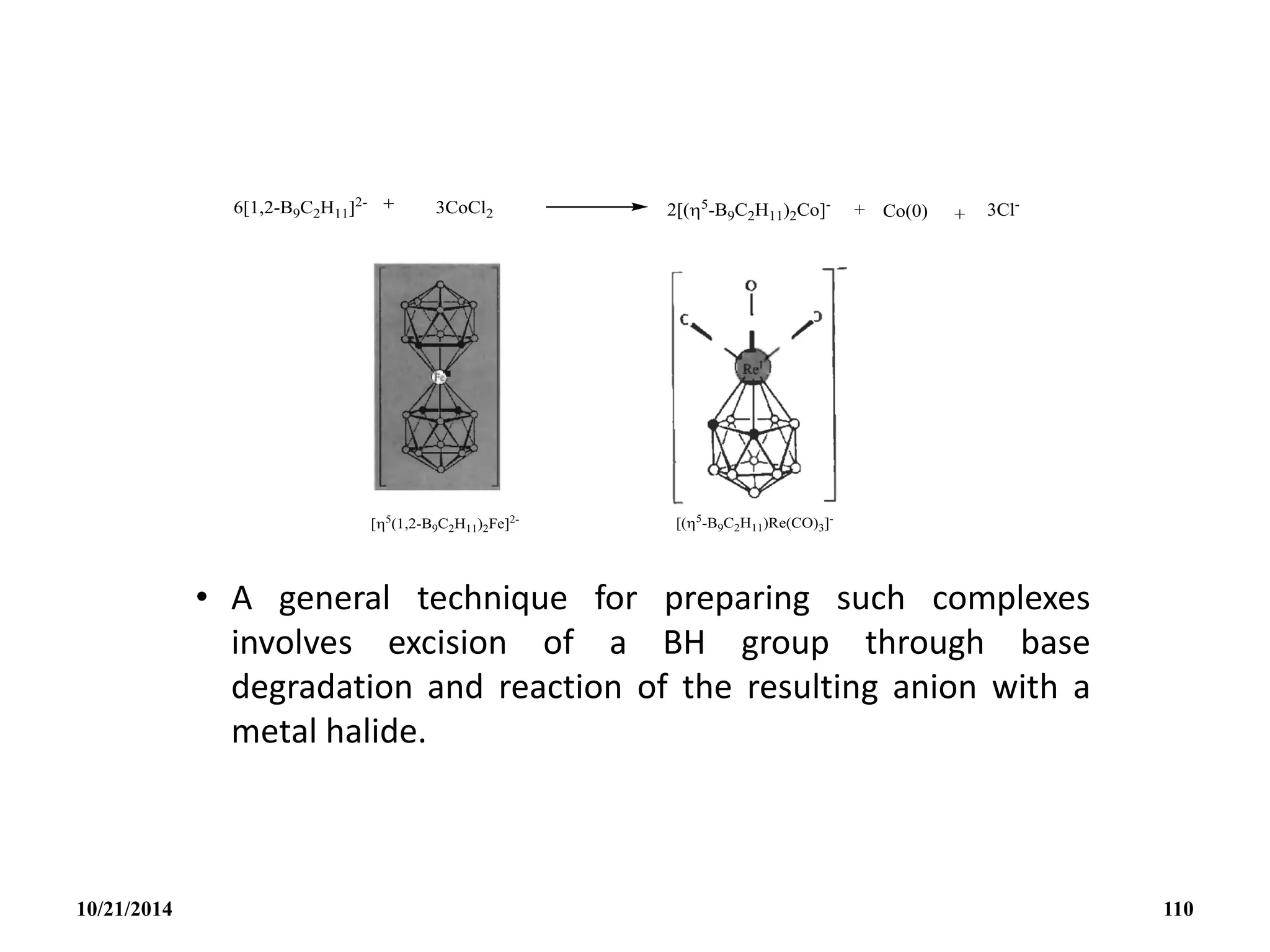
![(v) Subrogation of a {BH}
Subrogation of a {BH} unit in B5H9 by an ‘isoelectronic’
organometallic group such as {Fe(5-C5H5)} can occur and this
illustrates the close interrelation between metalloborates,
metal-metal cluster compounds and organometallic complexes
in general. Eg. [1-{Fe(CO)3}B4H8] ; [1-{Co(5-C5H5)}B4H8] ; [2-
{Co(5-C5H5)}B4H8] .
10/21/2014 80](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-80-2048.jpg)

![(a) Proton abstraction
• B10H14 can be titrated in aqueous/alcoholic media
as a monobasic acid : pka 2.70.
• Proton abstraction can also be effected by other
strong bases such as H-, OMe-, NH2
- etc.
• X-ray studies on [Et3NH]+[B10H13]- established that
the ion is formed by loss of a bridge proton as
expected and this results in considerable
shortening of the B(5)-B(6) distance from 179 pm
in to 165 pm in B10H13
-.
10/21/2014 85](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-85-2048.jpg)
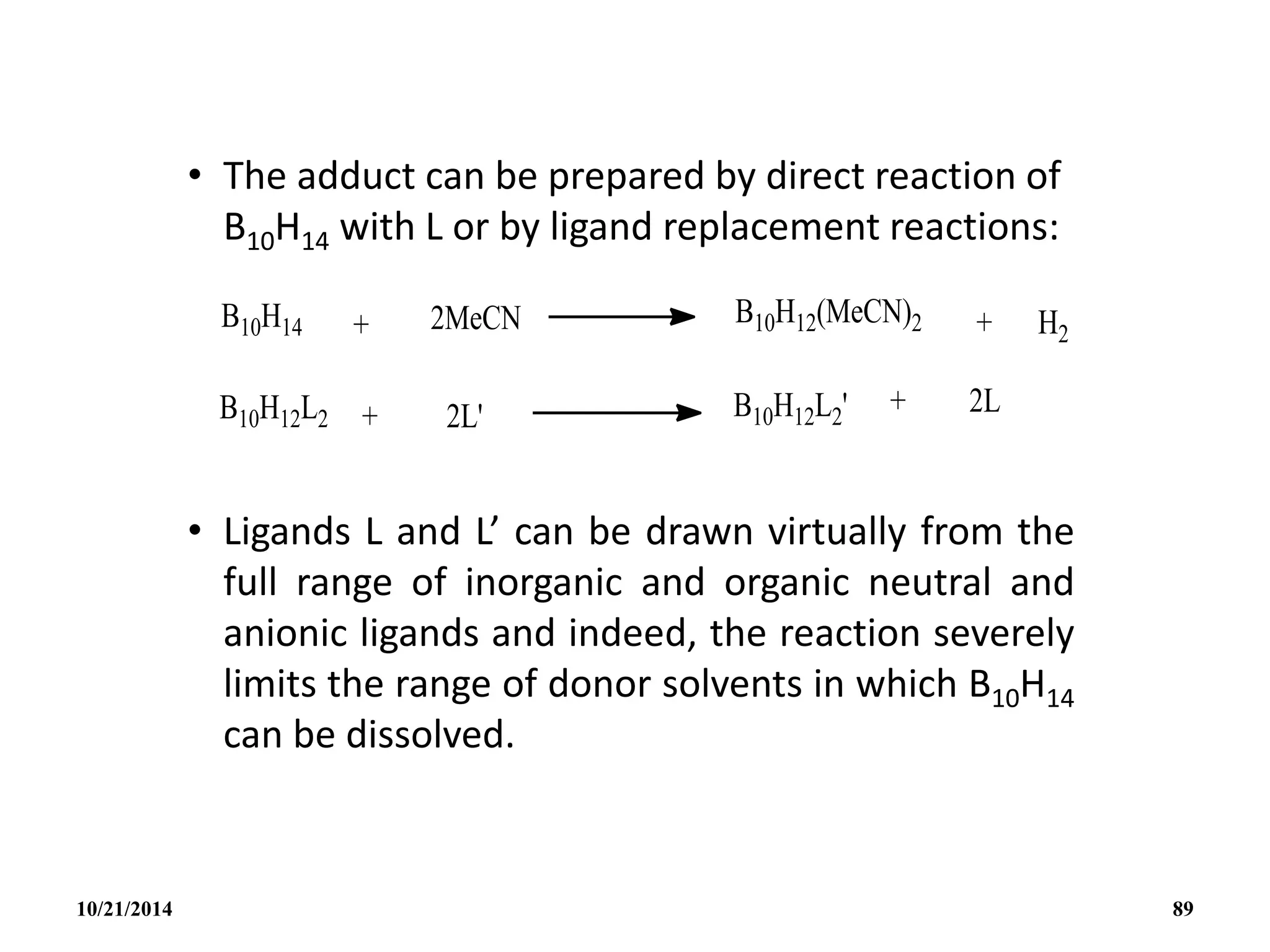
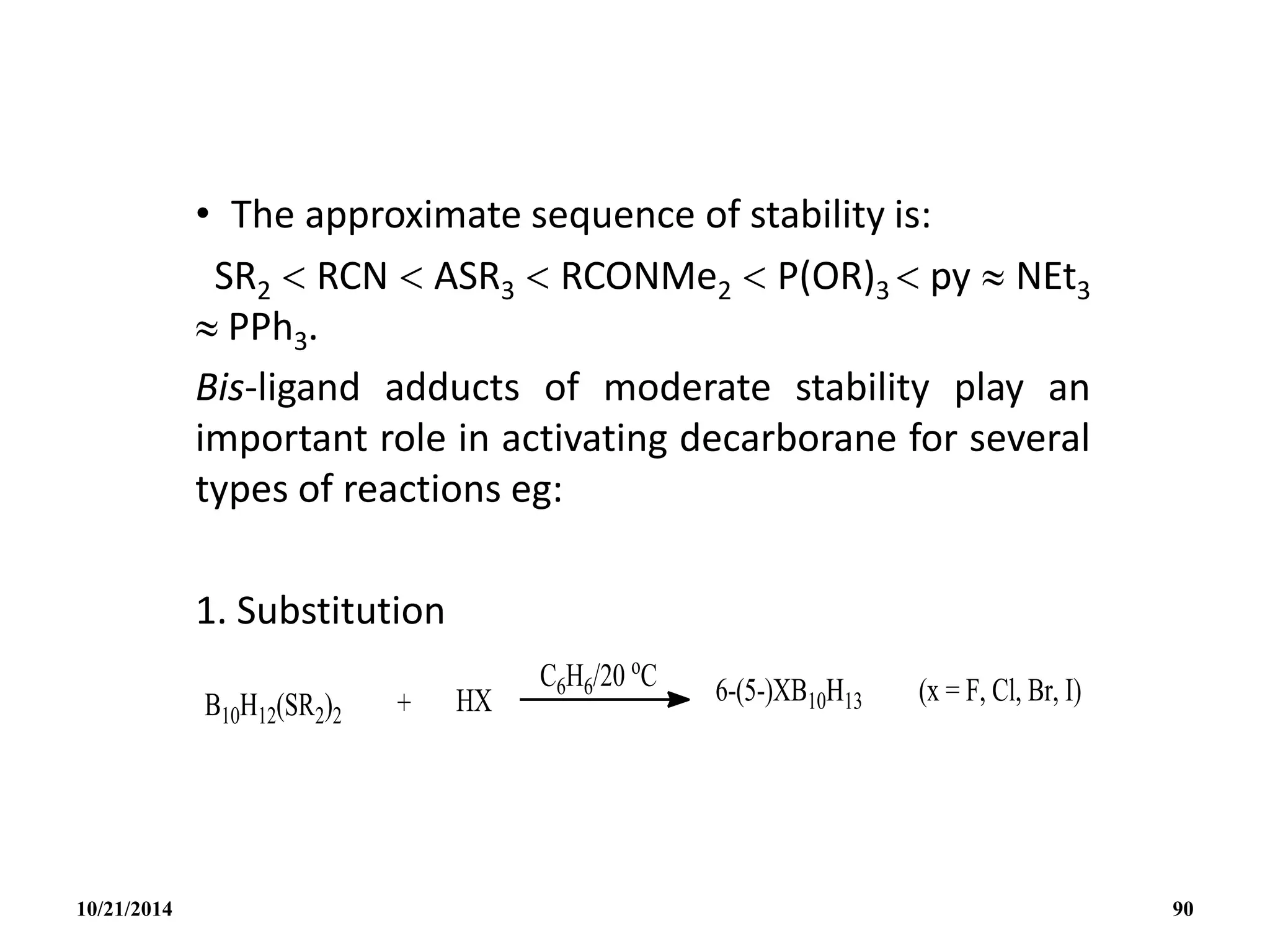
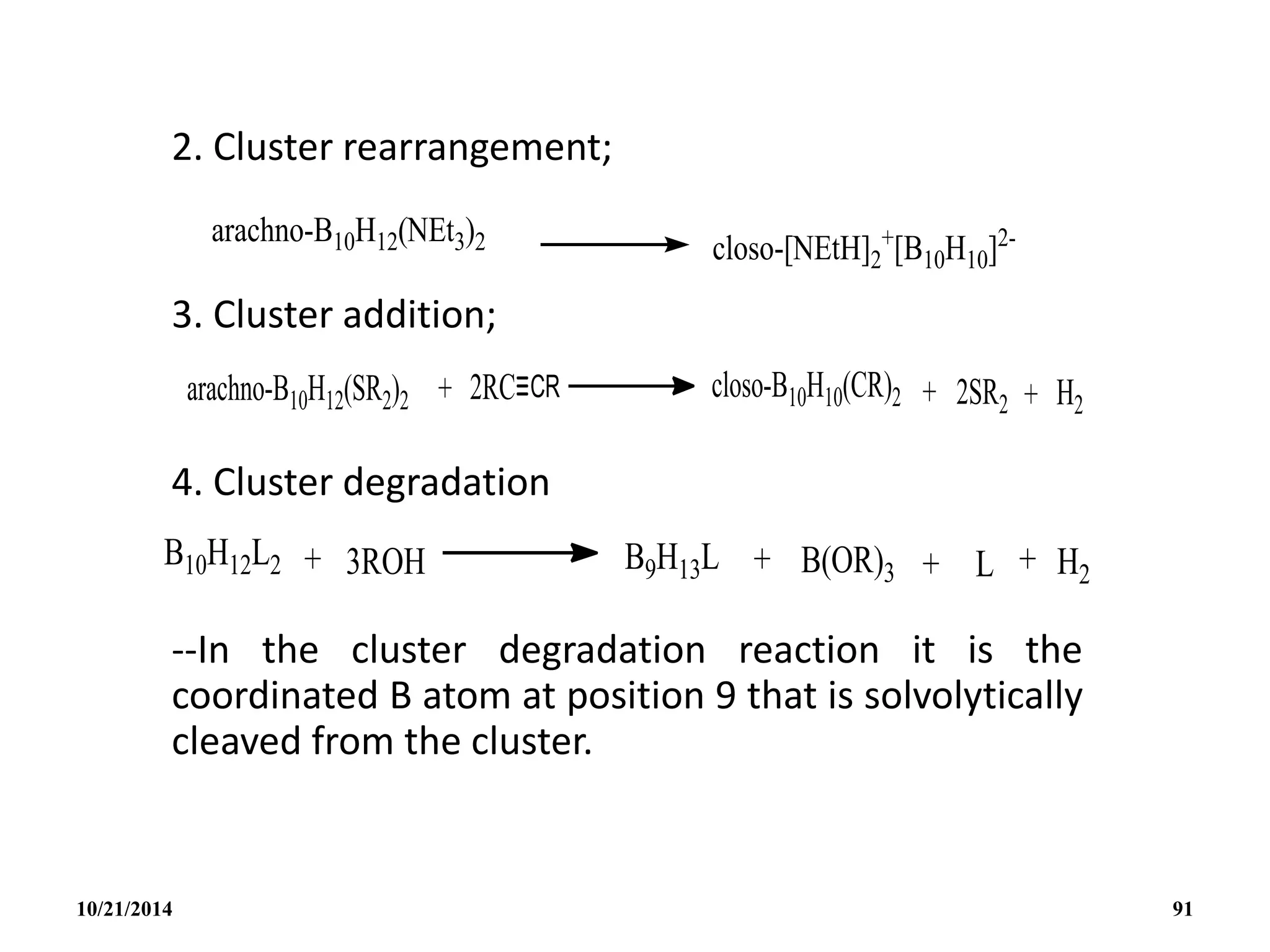
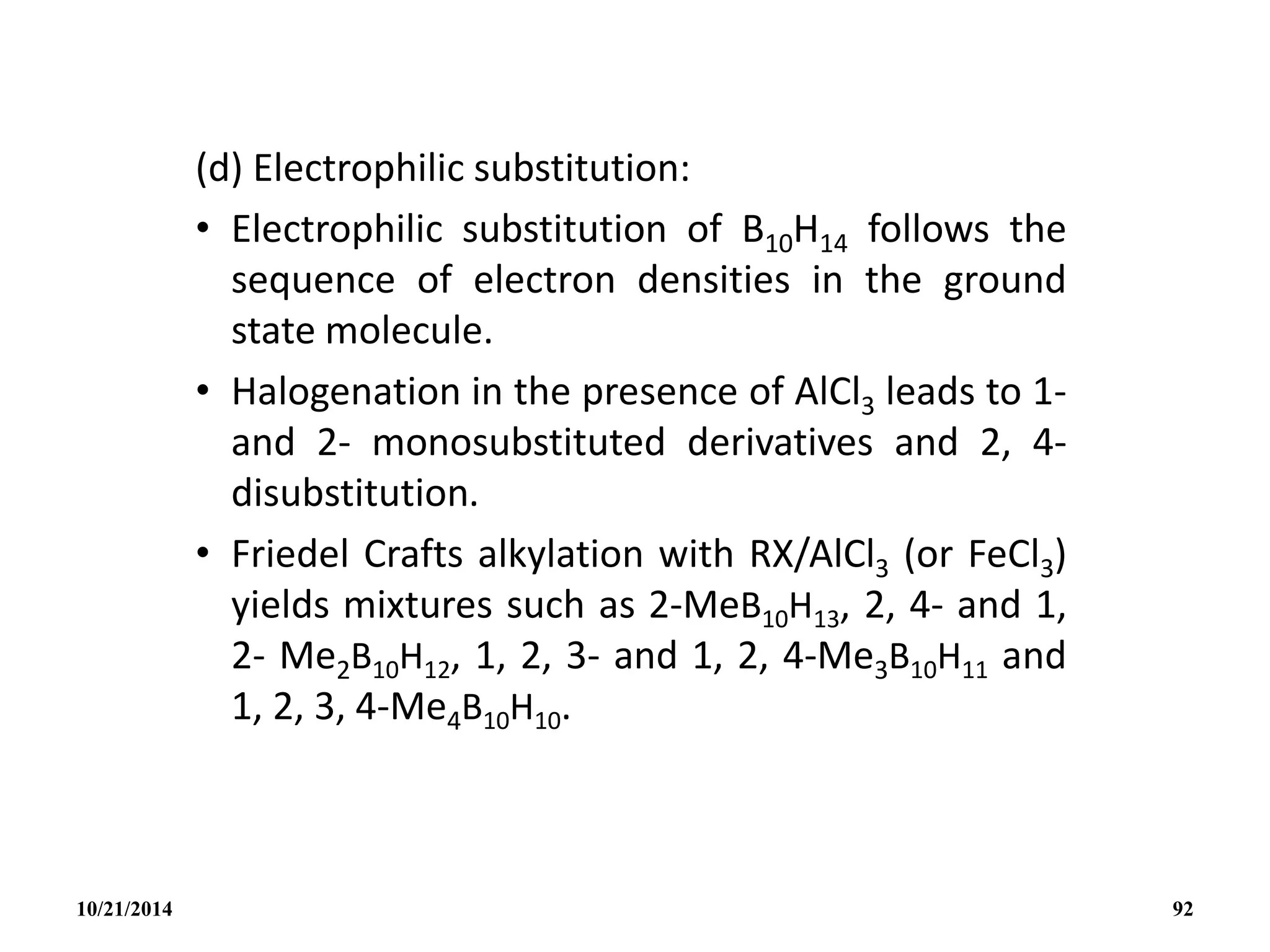
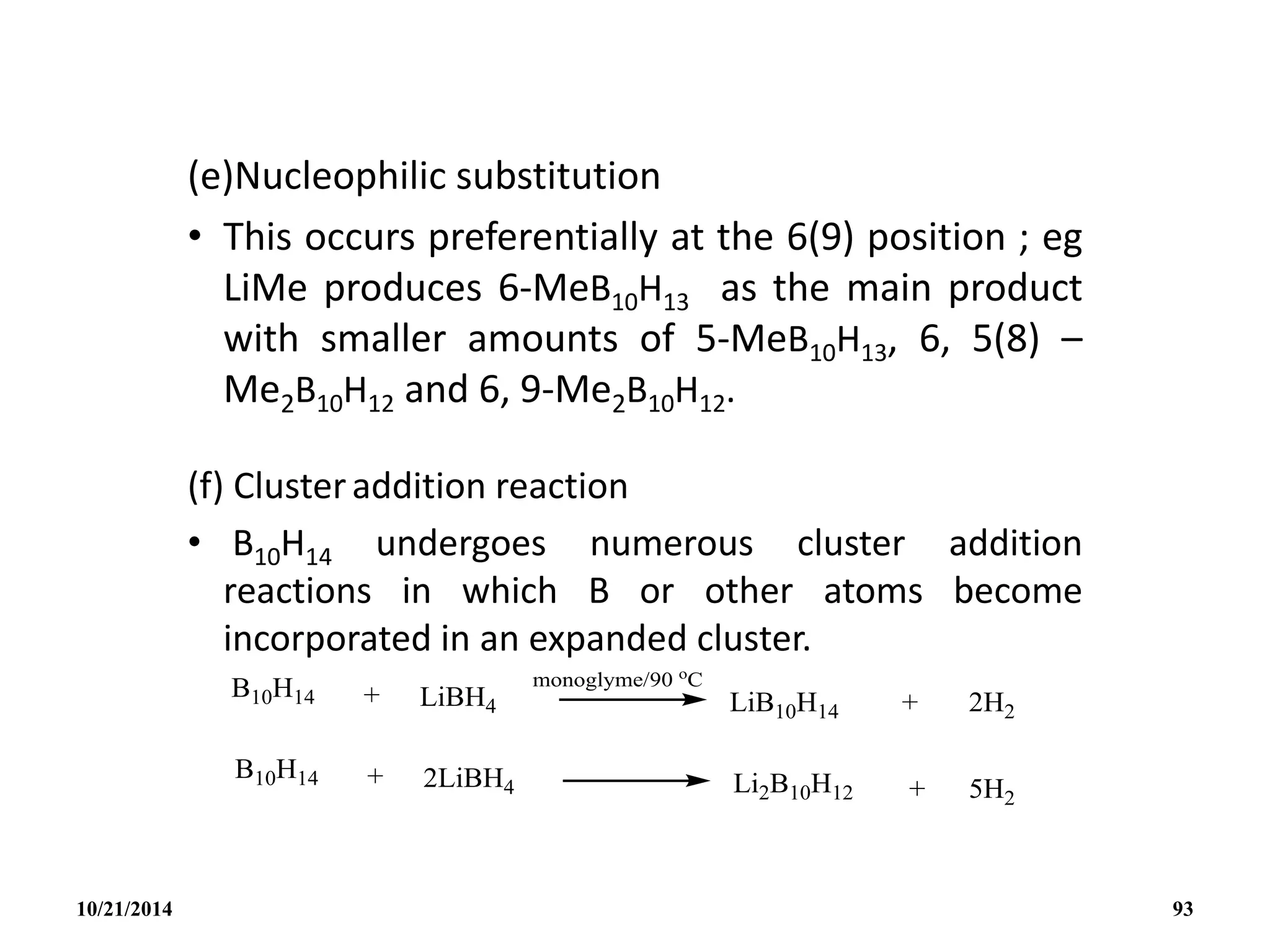
![• A more convenient high yield synthesis of B10H12
2-
is by the direct reaction of amine boranes with
B10H14 in the absence of solvents.
• Heteroatom cluster addition reactions are
exemplified by the following:
• The structure of the highly reactive anion
[AlB10H14]- is thought to be similar to the nido-
B11H14
- with one facial B atom replaced by Al.
10/21/2014 94](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-94-2048.jpg)
![• Many other complexes [M(B10H12)2]2- and [L2M(B10H12)]
are known with similar structures except that, where M
= Ni, Pd, Pt, the coordination about the metal is
essentially square-planar rather than pseudo-
tetrahedral as for Zn, Cd and Hg.
10/21/2014 96](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-96-2048.jpg)
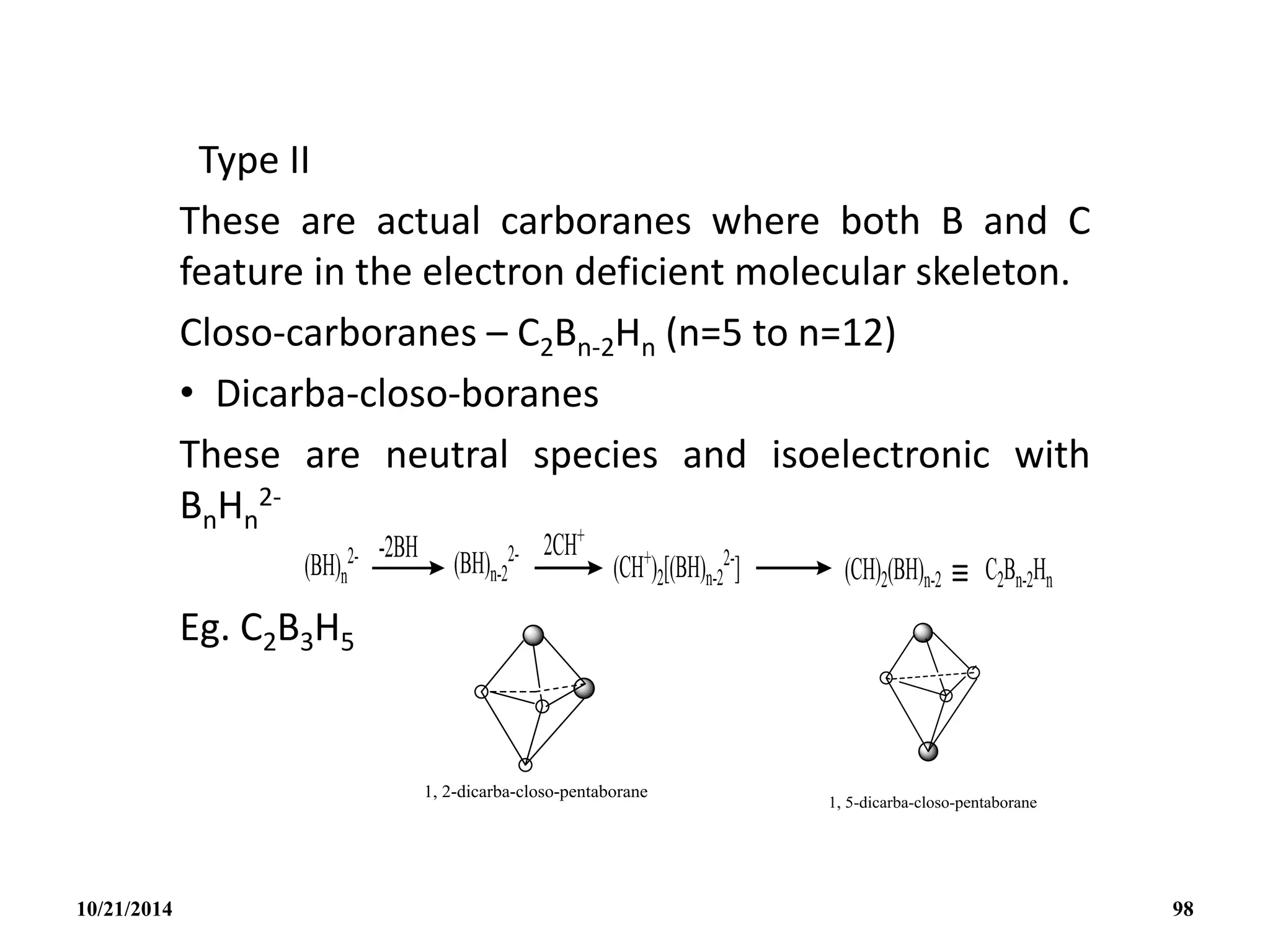
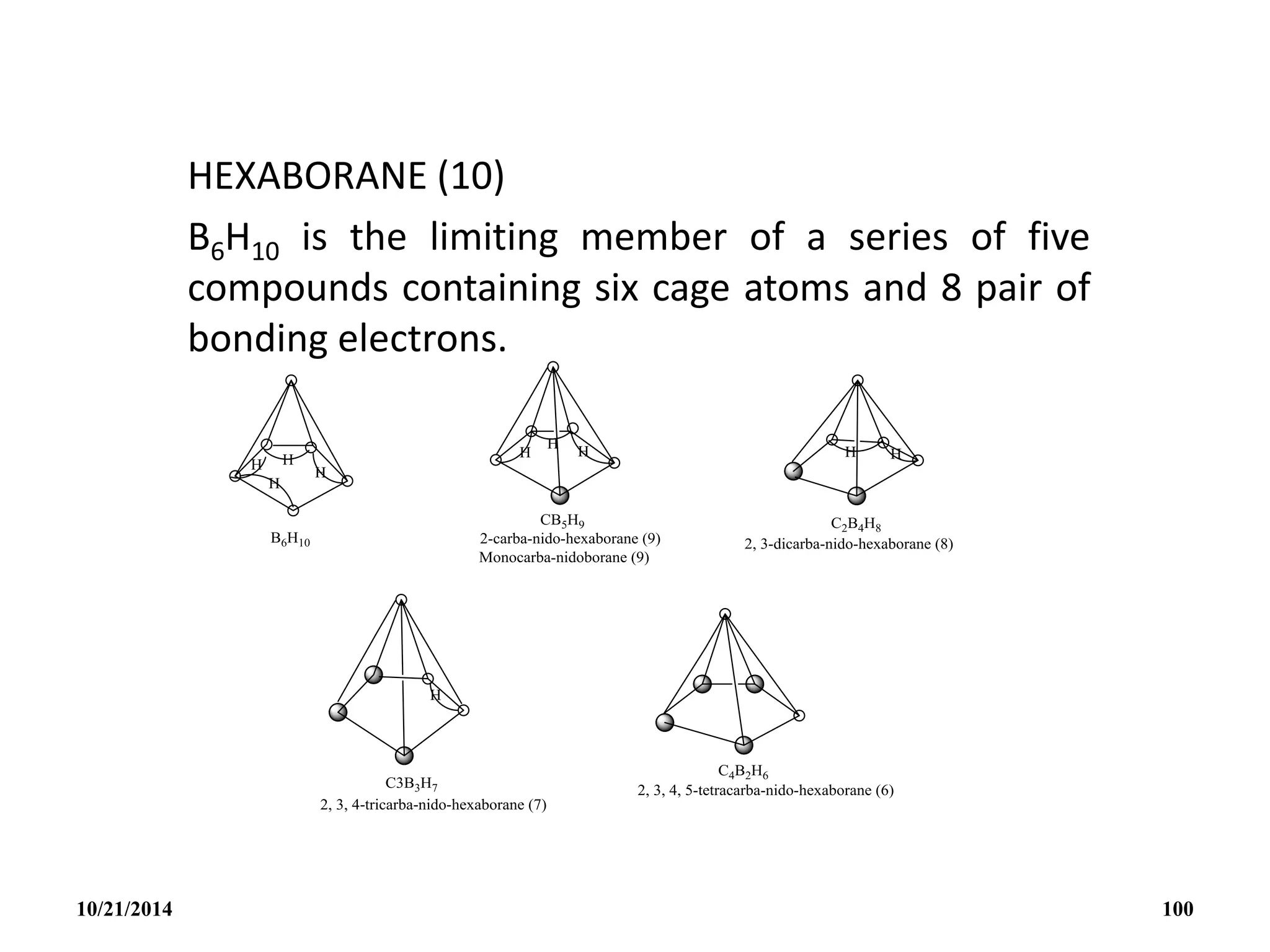
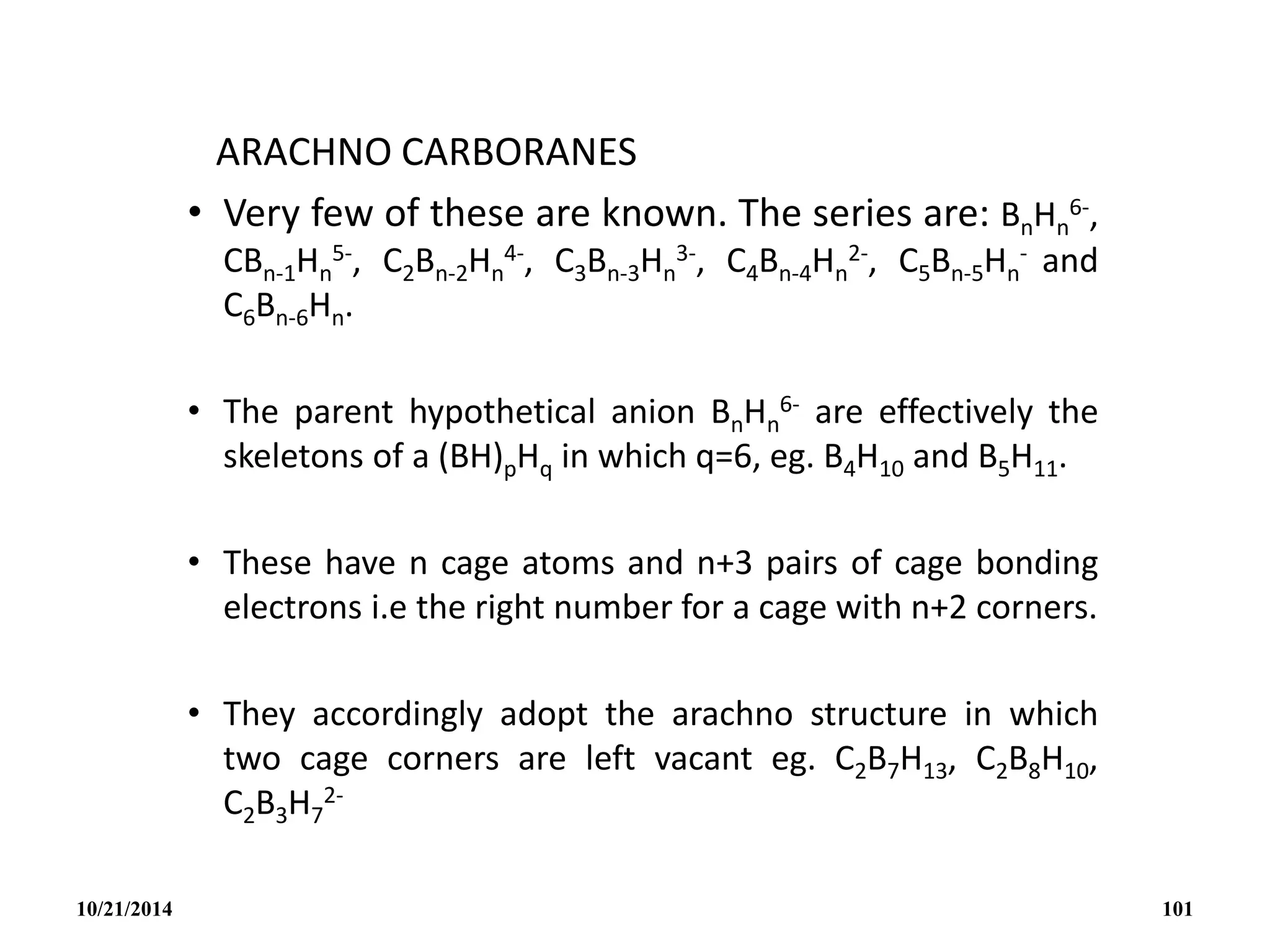
![C2B4H6
NIDO CARBORANES [C2Bn-2Hn
2-]
These have n cage atoms and n+2 pairs of cage bonding
electrons and n+1 corners.
Structurally they adopt an incomplete cage structure, nido or
nest structure i.e one cage corner is left vacant though when
obtained as metal salts, the metal cation may occupy the
vacant site in the crystal.
Isoelectronic species which might all be expected to have the
nido structures are: BnHn
4-, CBn-1Hn
3-, C2Bn-2Hn
2-, C3Bn-3Hn
-,
C4Bn-4Hn.
10/21/2014 99](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-99-2048.jpg)



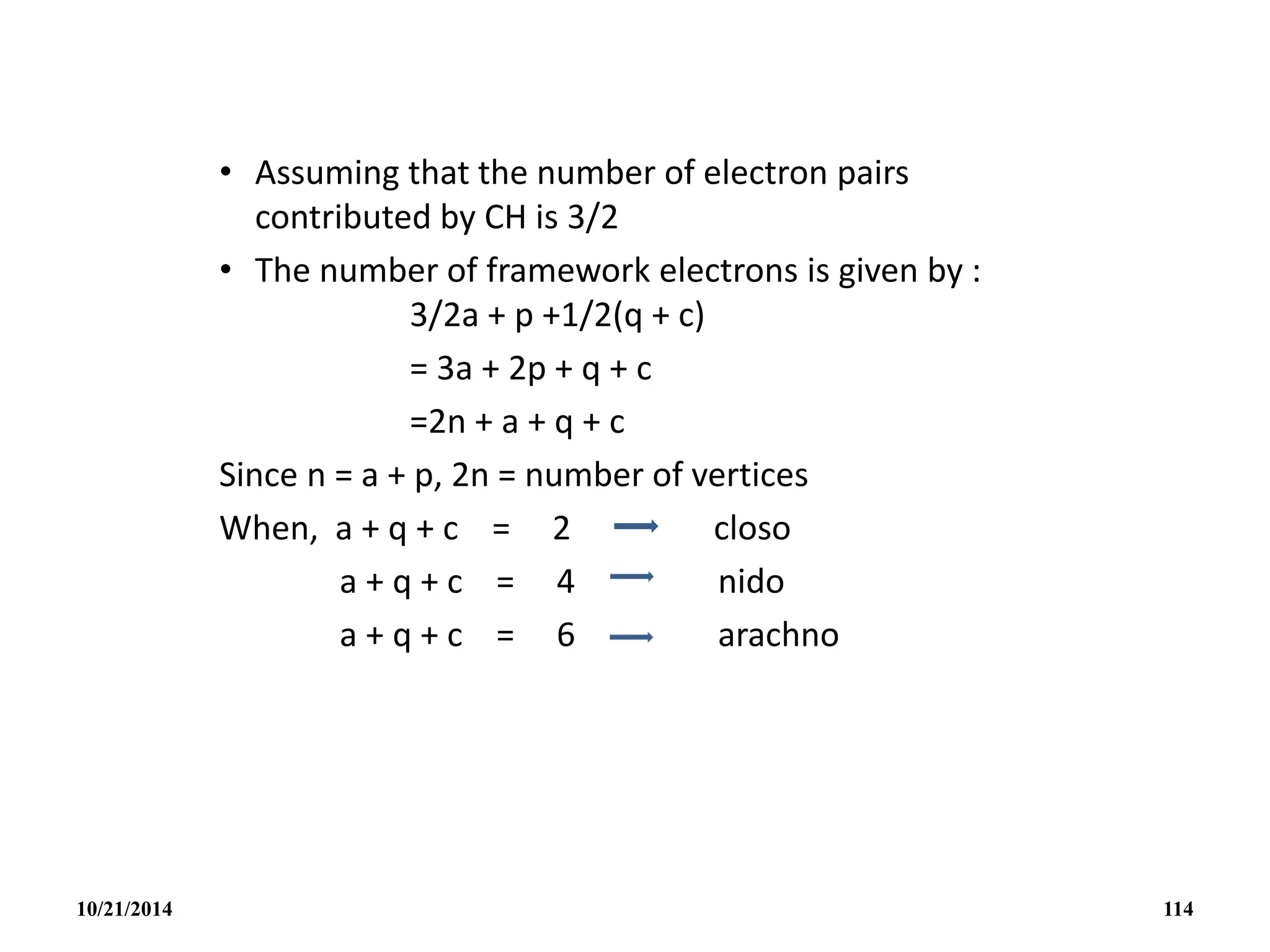
![• Eg. [B6H6]2-
(BH)6
2- = (6x3e-s) + (6x1e-) + 2e-s = 26e-s
6B-H = (6x2e-s) = -12e-s
2n + a + q + c = 14e-s
2n = -12e-s
Closo a + q + c = 2e-s
• B5H11
(BH)5 = (5x3e-s) + (11x1e-) = 26e-s
5B-H = (5x2e-s) = -10e-s
2n + a + q + c = 16e-s
2n = -10e-s
Arachno a + q + c = 6e-s
10/21/2014 115](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-115-2048.jpg)
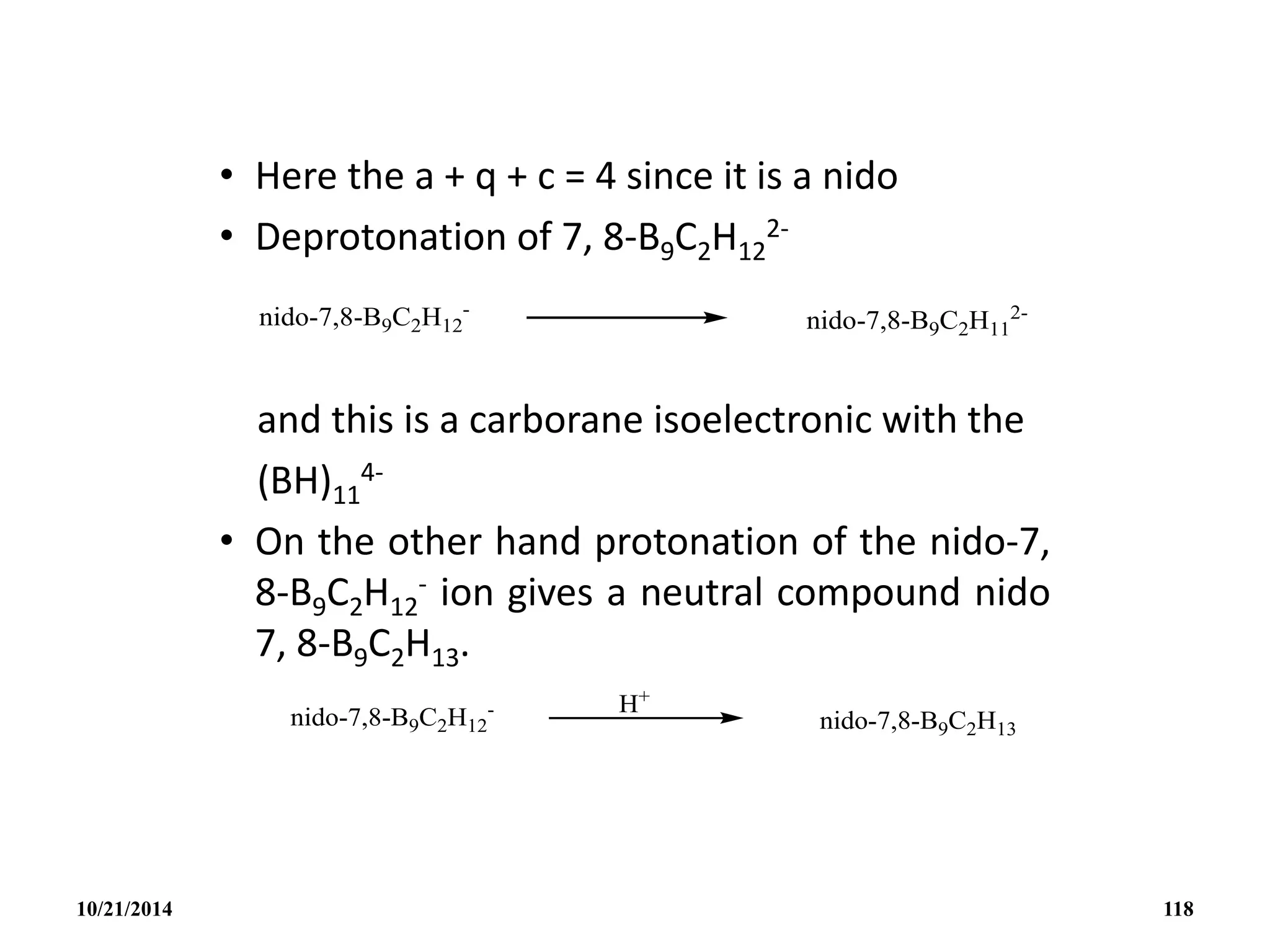
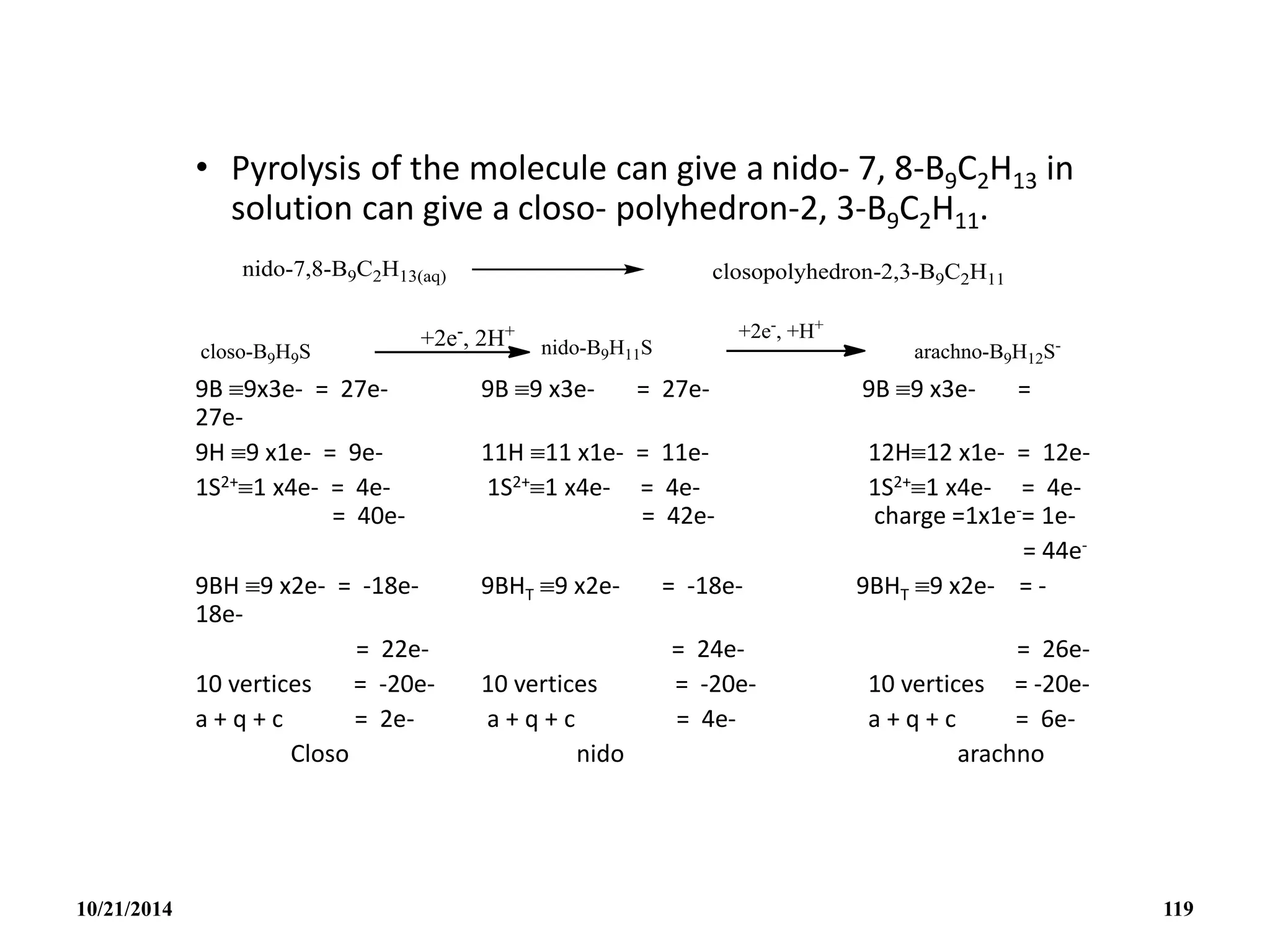
![• Eg. The closo-B4C2H6 may be generated from a
closo B6H6
2- by the removal of 2BH units and
adding 2CH+ groups or units.
• The formula of this ion 7, 8-B9C2H12
- anion may be
written as [(CH)2(BH)9H]- if we compare with [(BH)11H]3-.
The [(CH)2(BH)9H]- anion compared with [(BH)9H]3- with
2CH+ unit replacing 2BH units and with a proton H+
stitching up part of the opened face.
10/21/2014 117](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-117-2048.jpg)
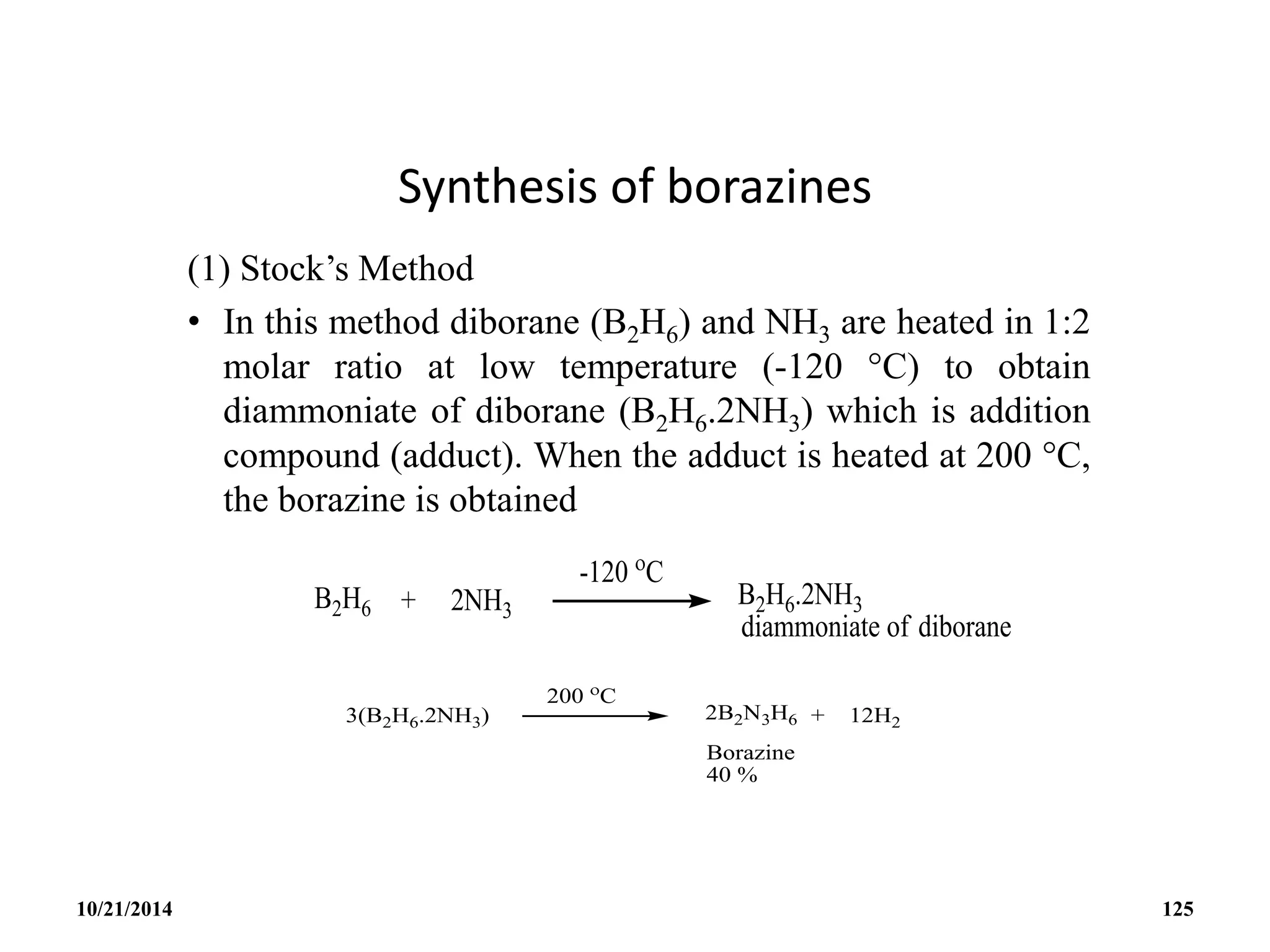
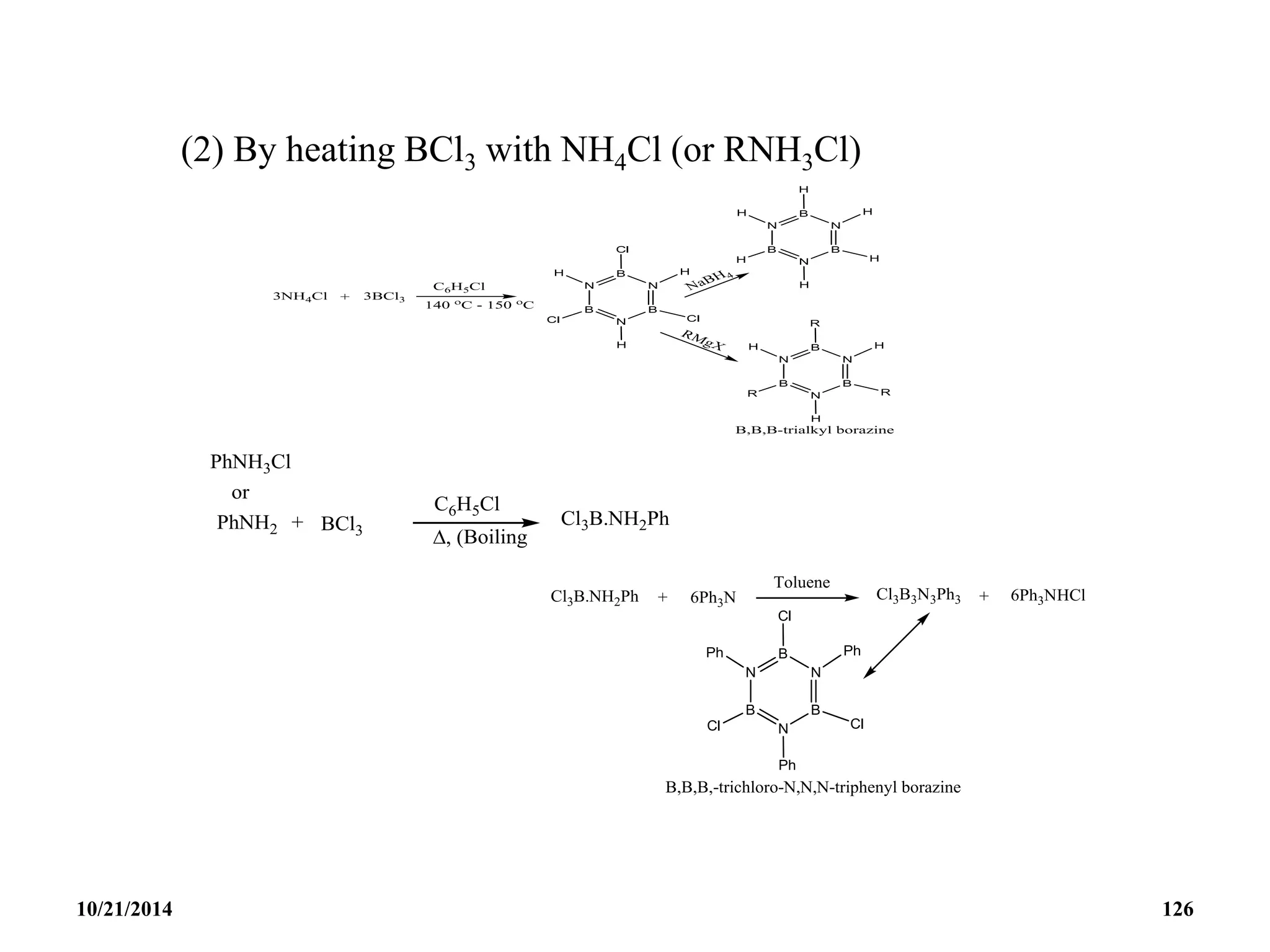
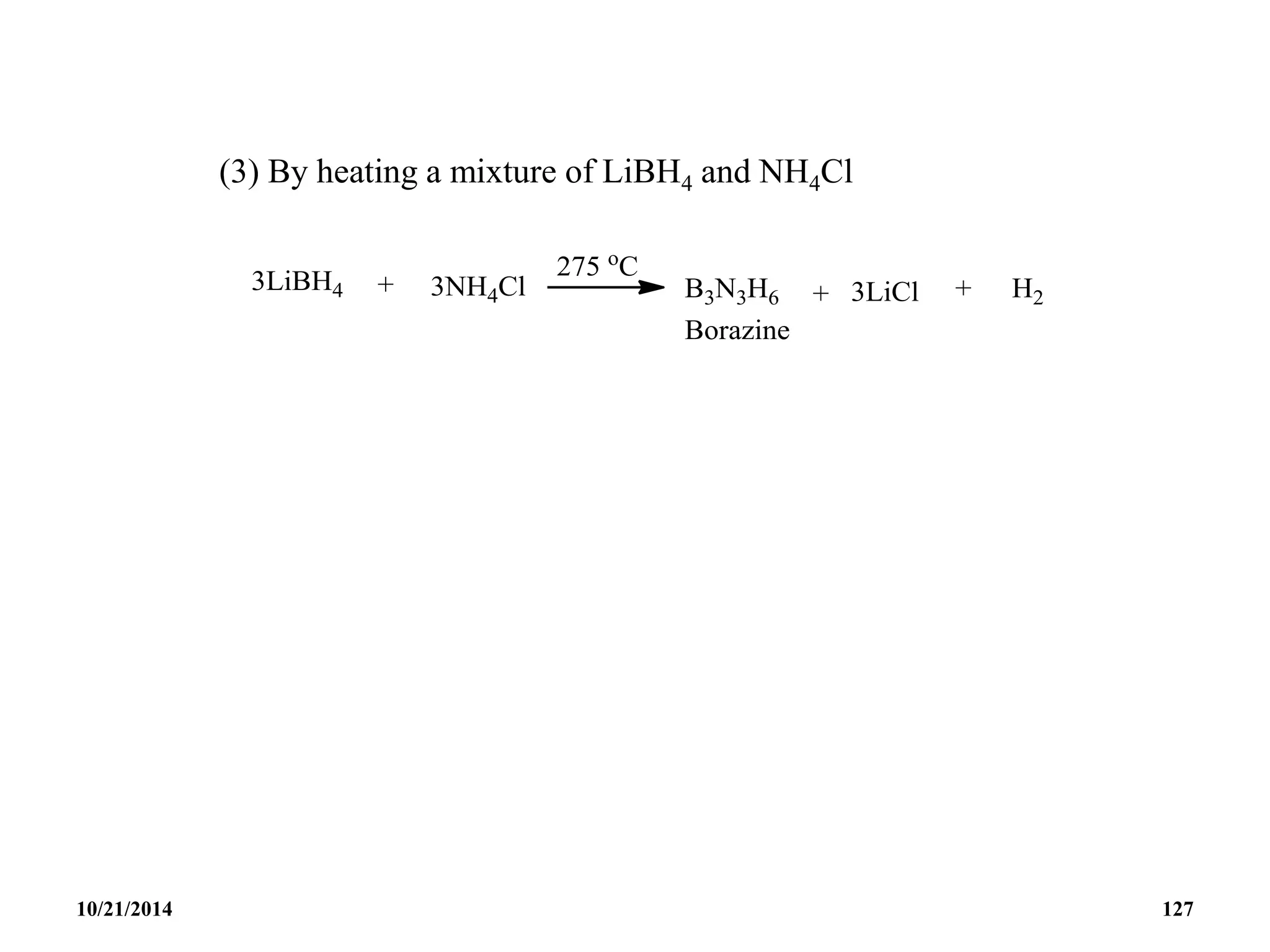
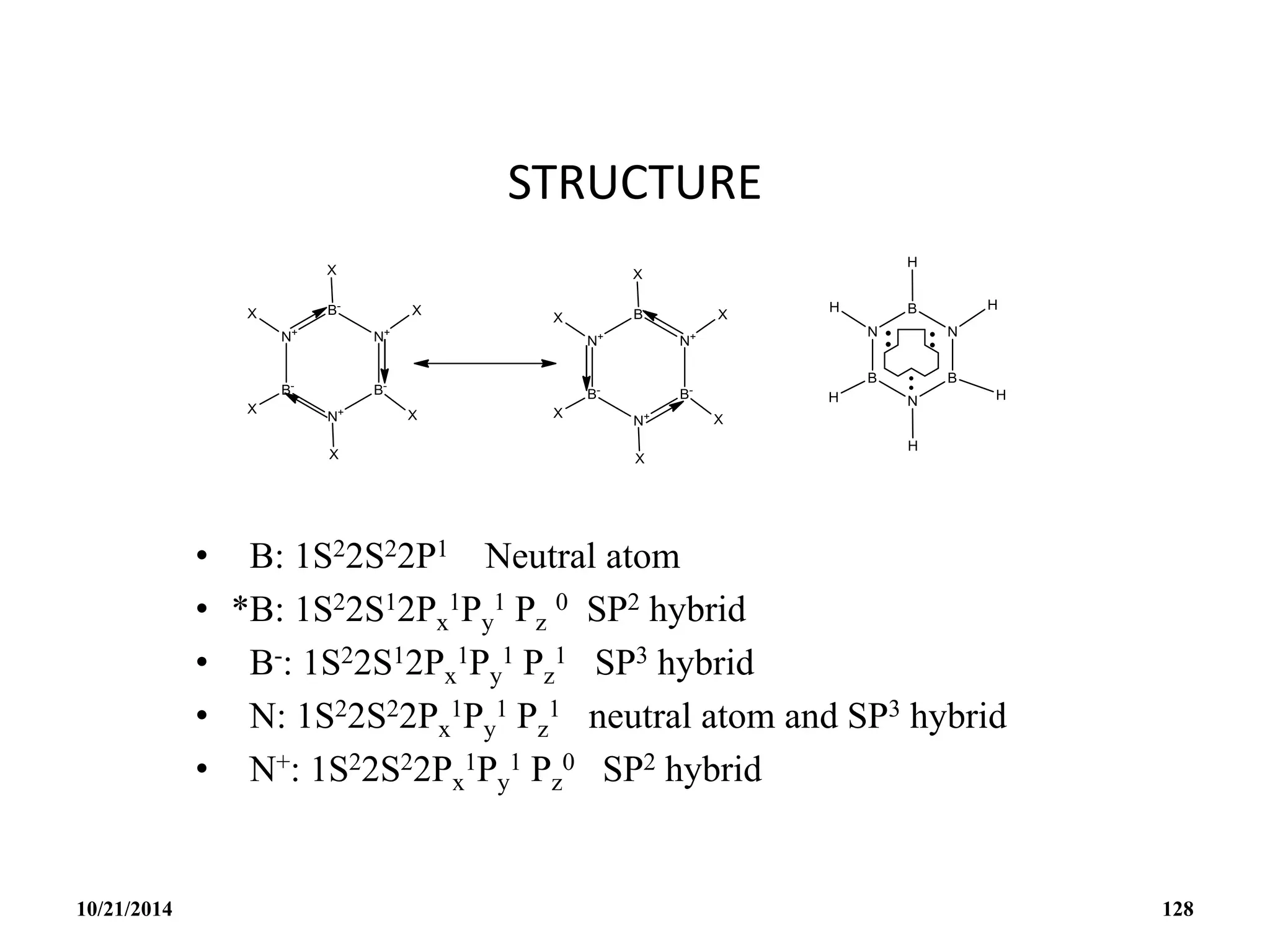


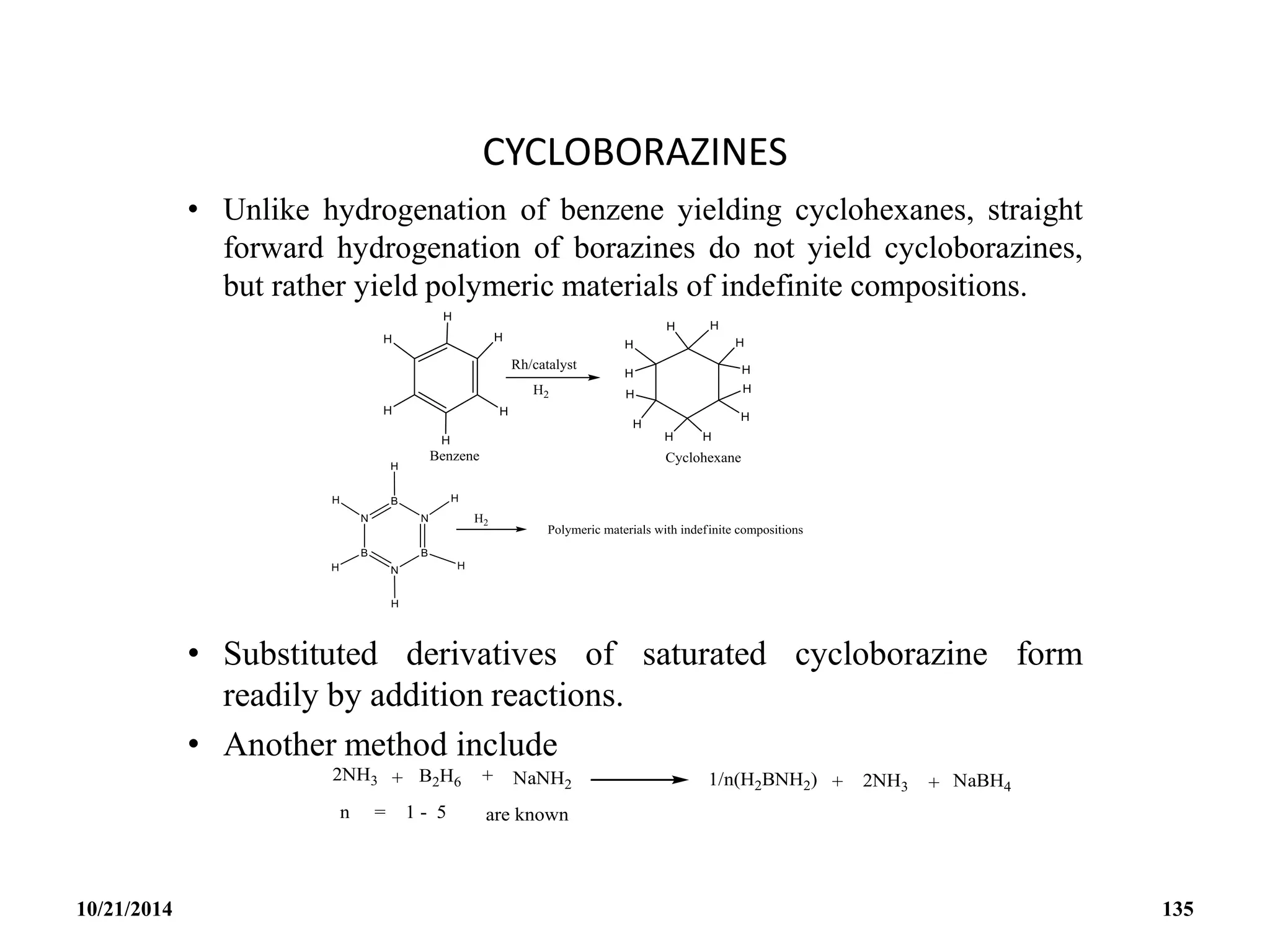
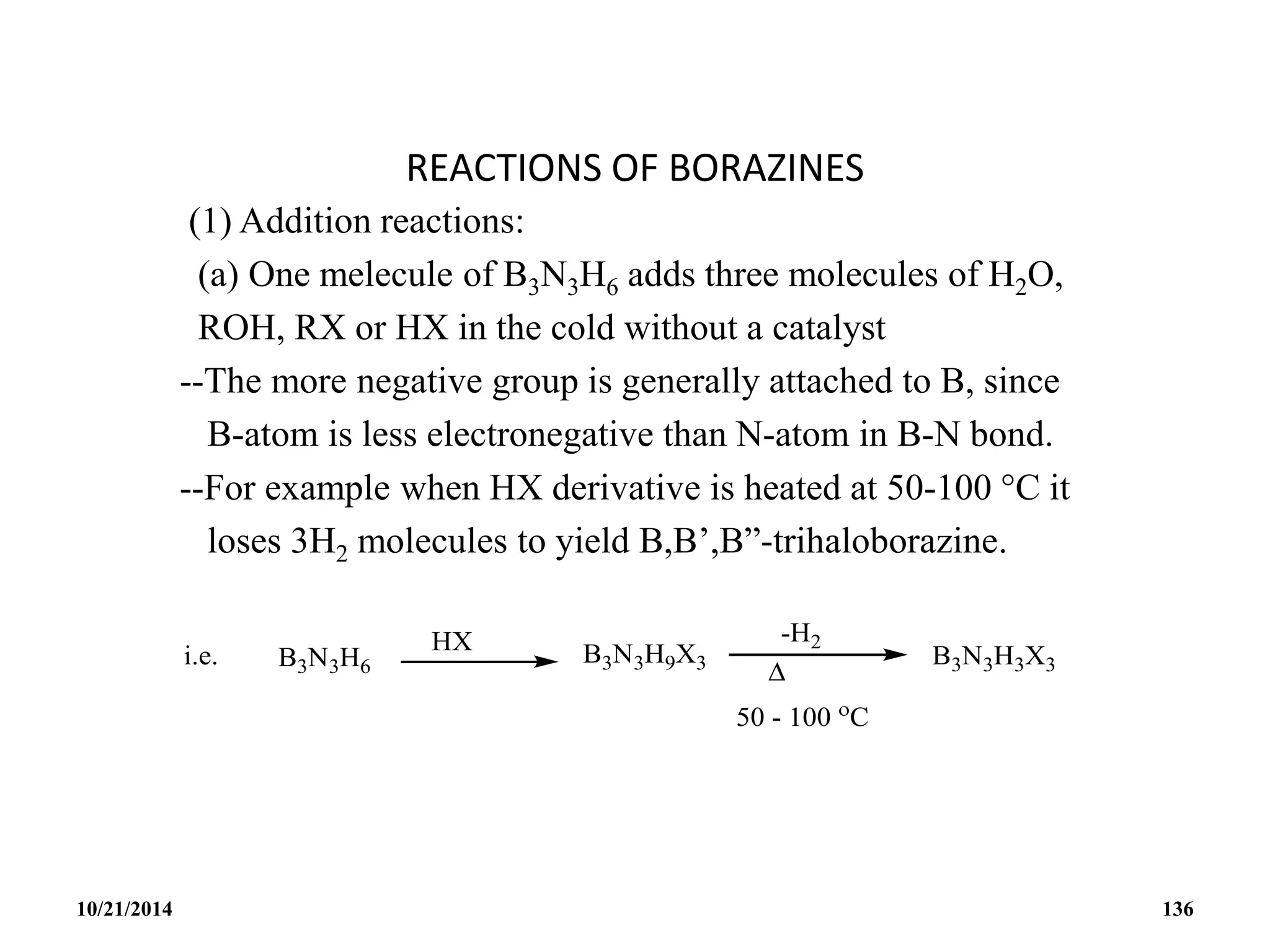
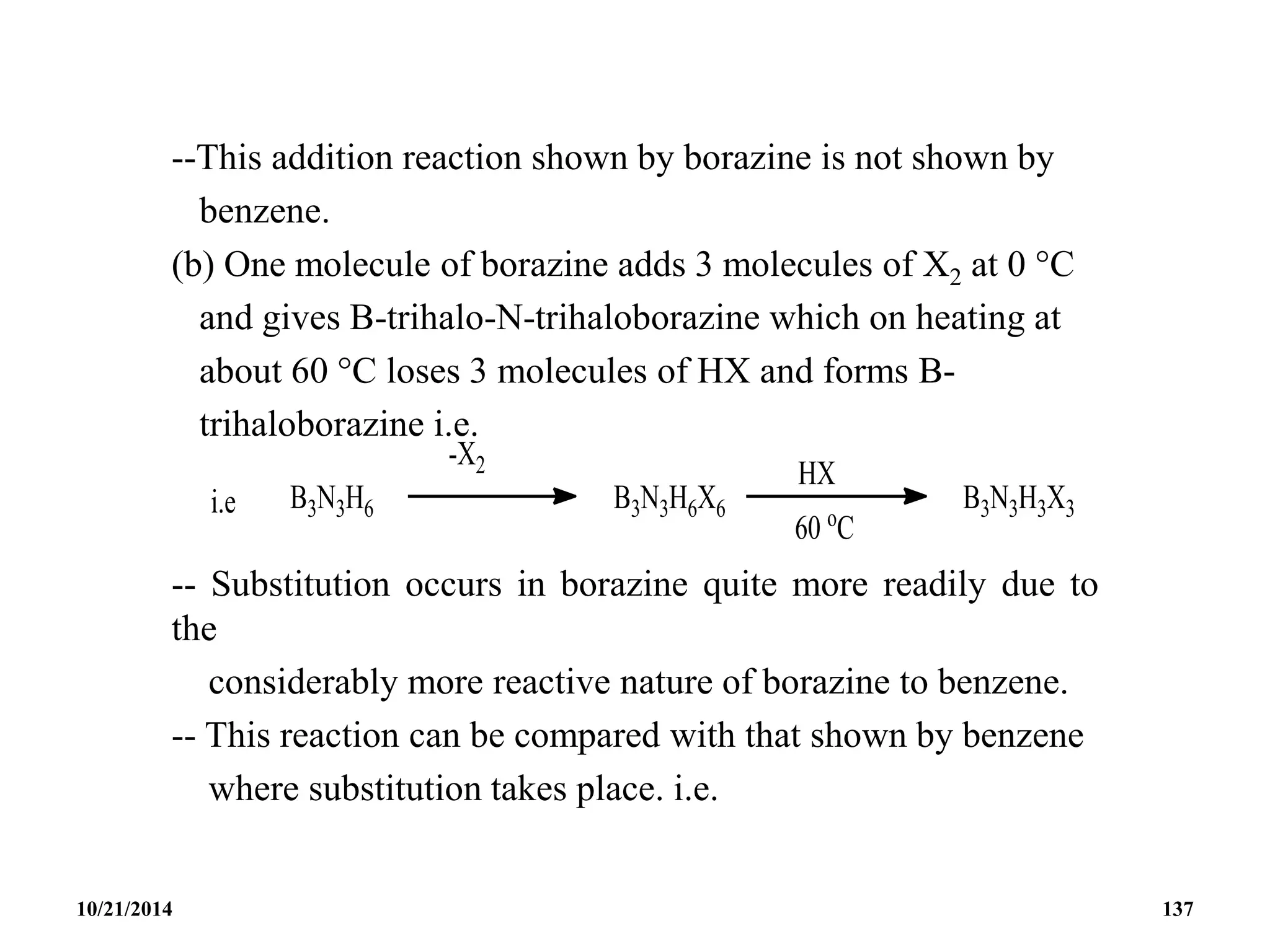
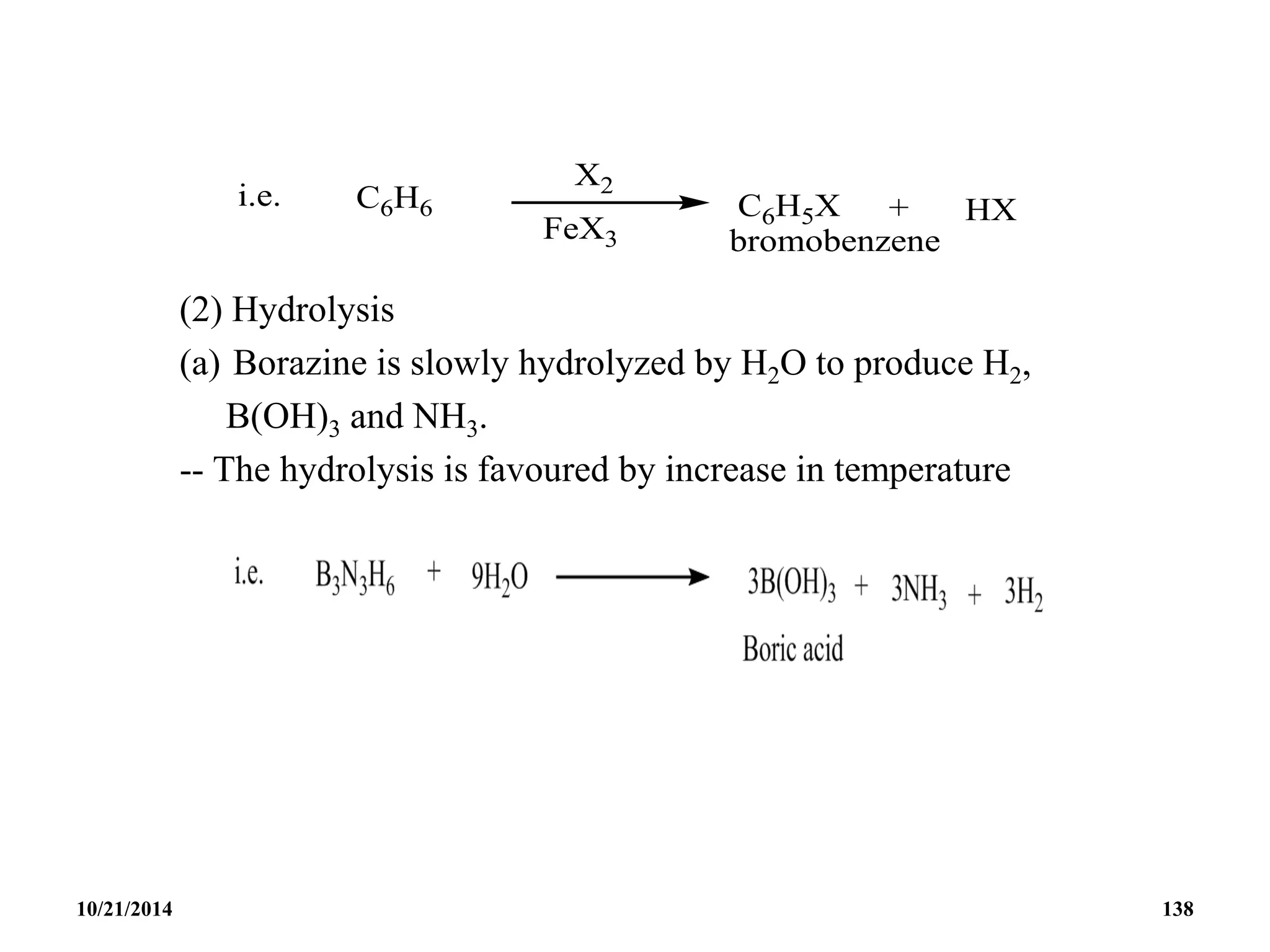
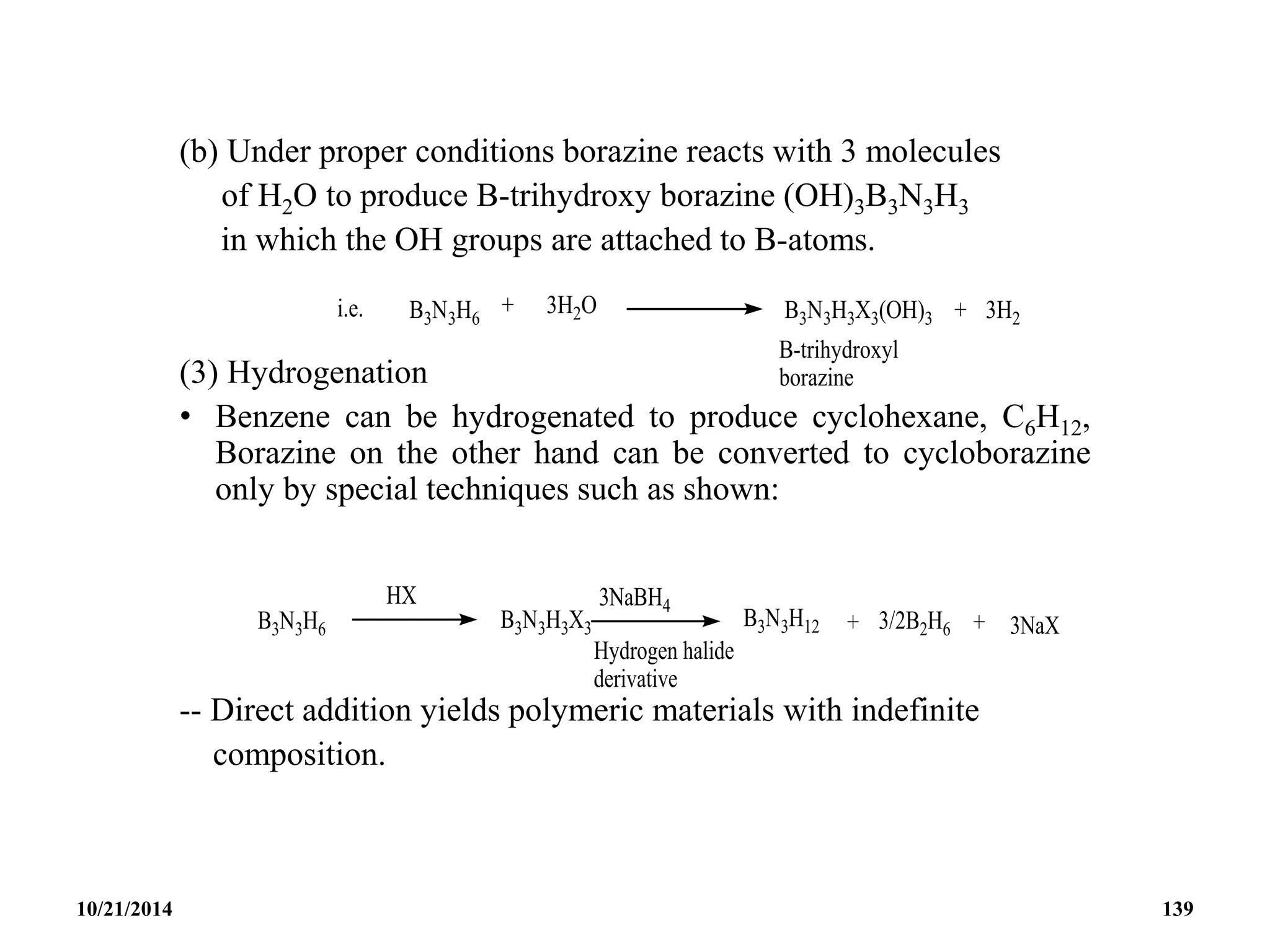
![BOROXINE
• Iso-electronic with borazine is boroxine H3B3O3 which is
formulated as [XBO]3
• Boroxine is planar but has less even –delocalization than
borazine and possess a six membered ring.
• Boroxine can be prepared by the explosive oxidation of
B2H6 or B5H9, or high temperature hydrolysis of boron.
• Although this compound is thermodynamically unstable at
room temperature with respect to B2H6 and boric oxide,
nevertheless it can be characterized.
10/21/2014 140](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-140-2048.jpg)
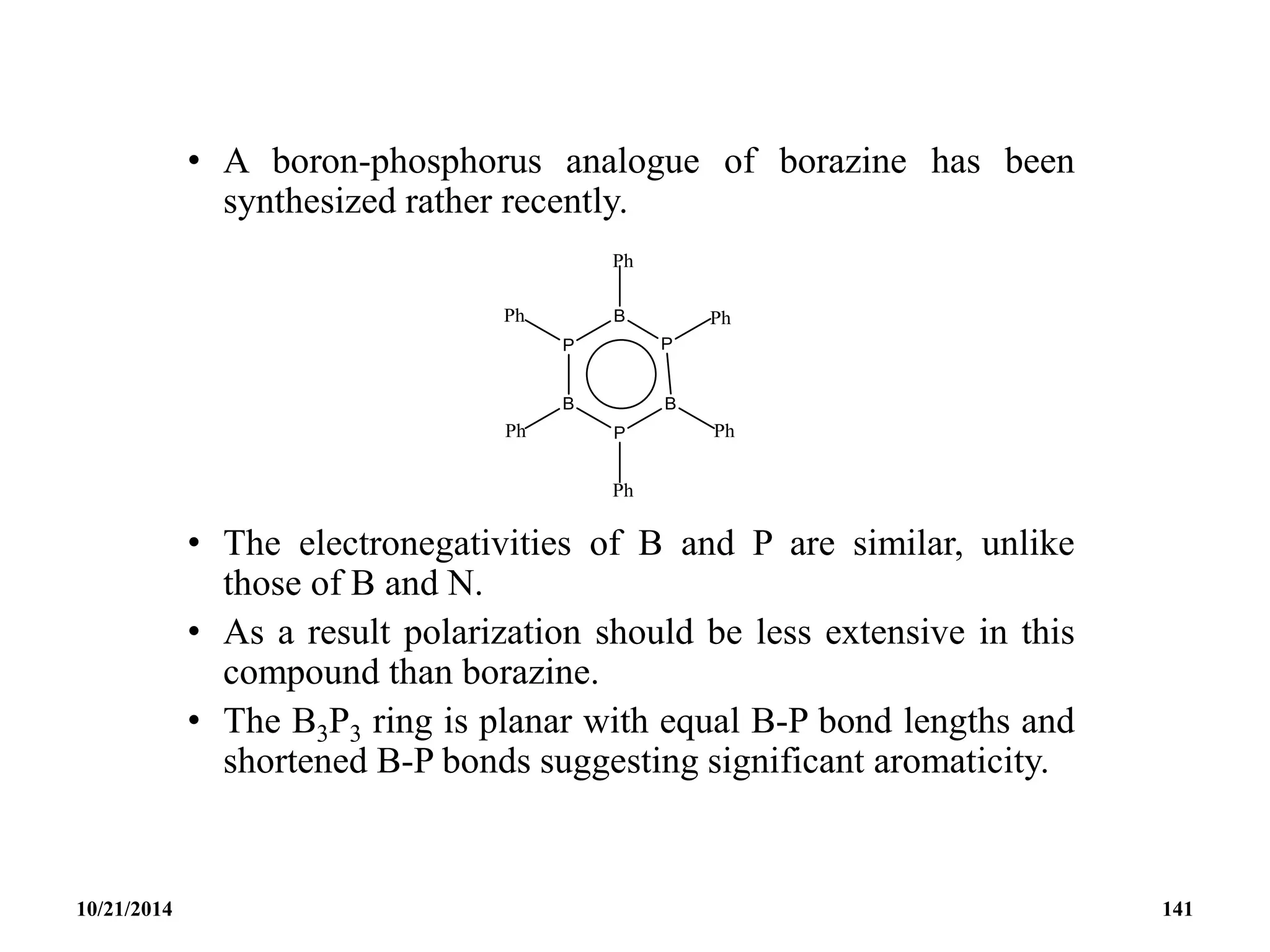
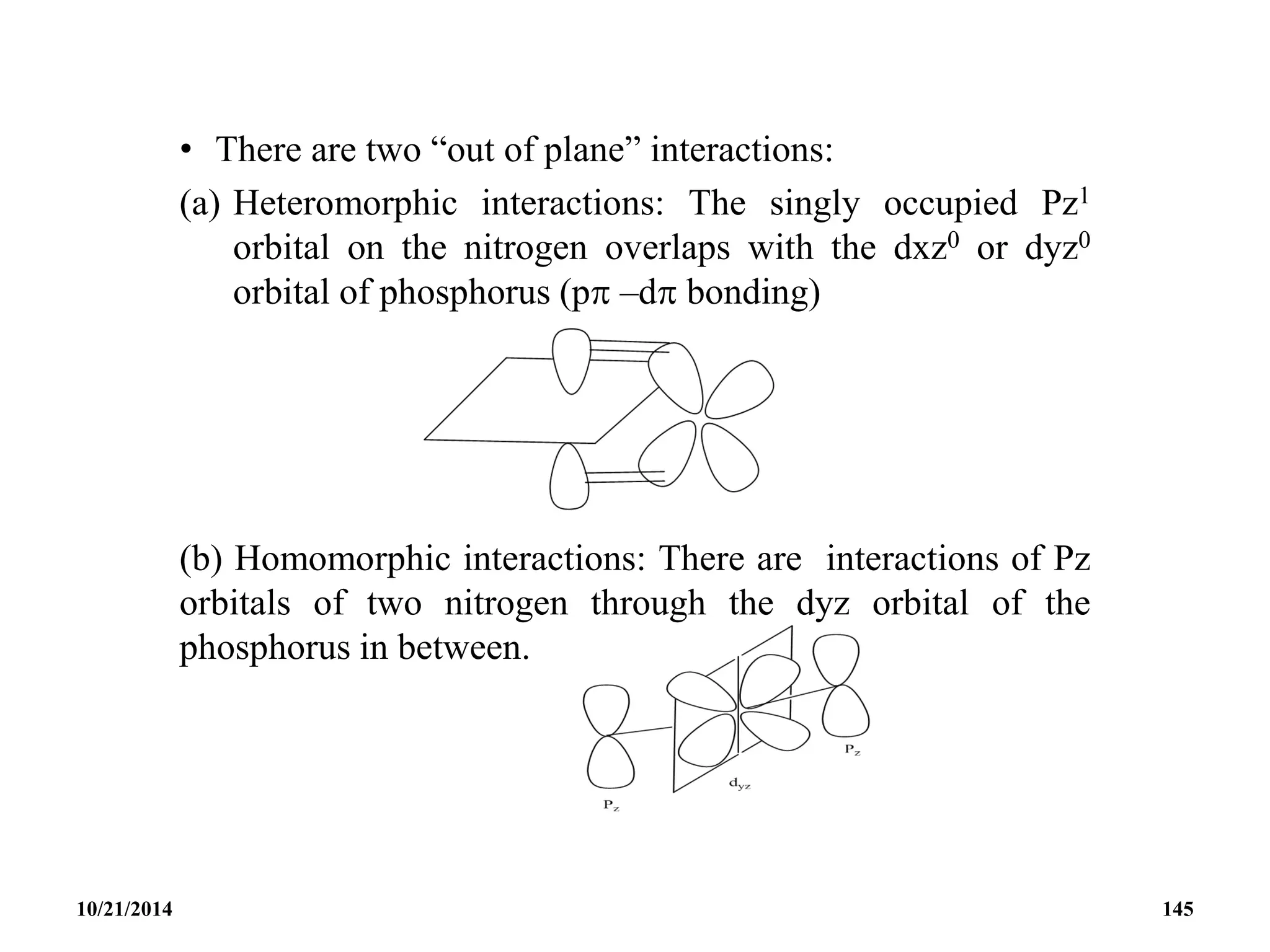
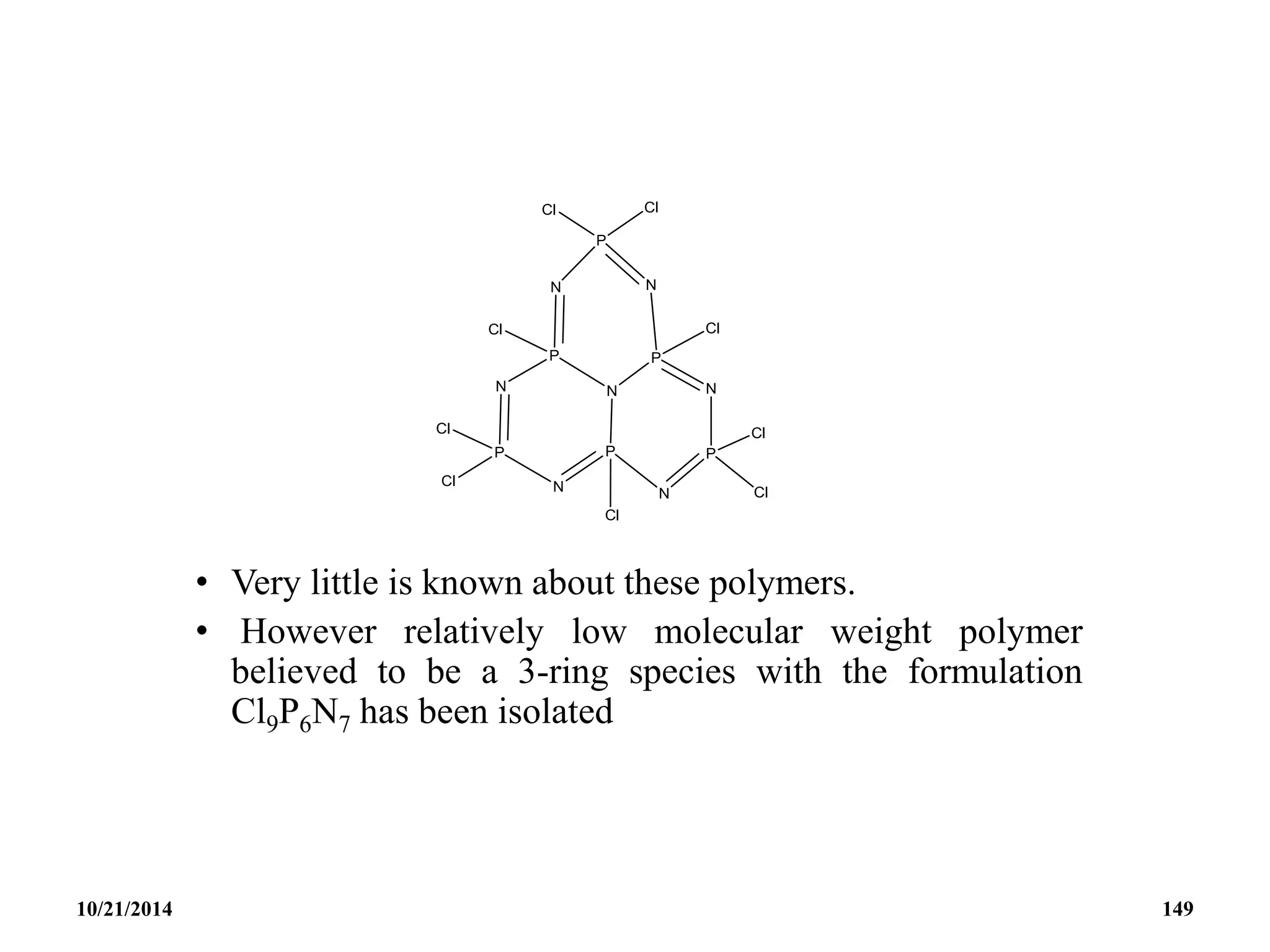
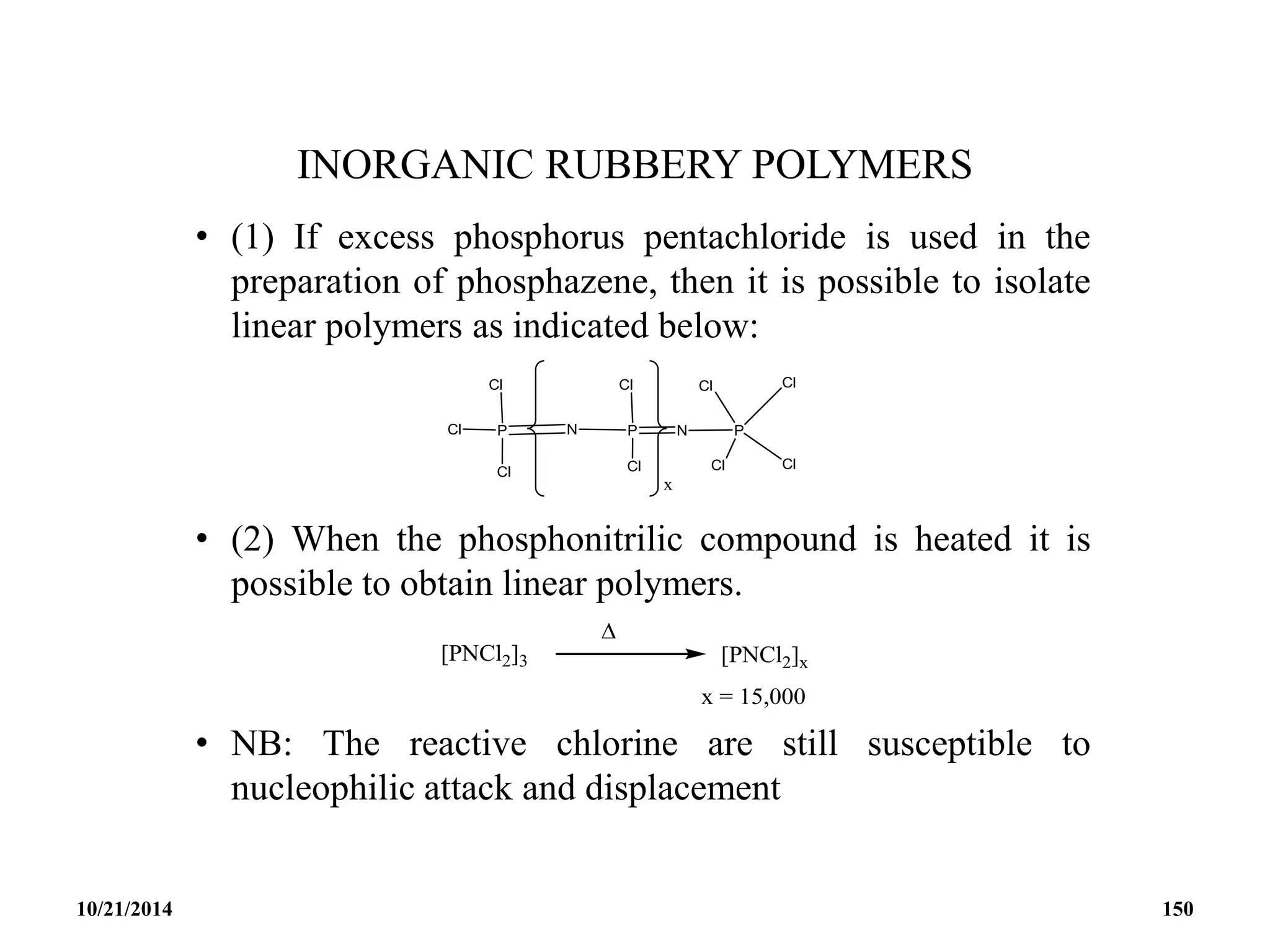
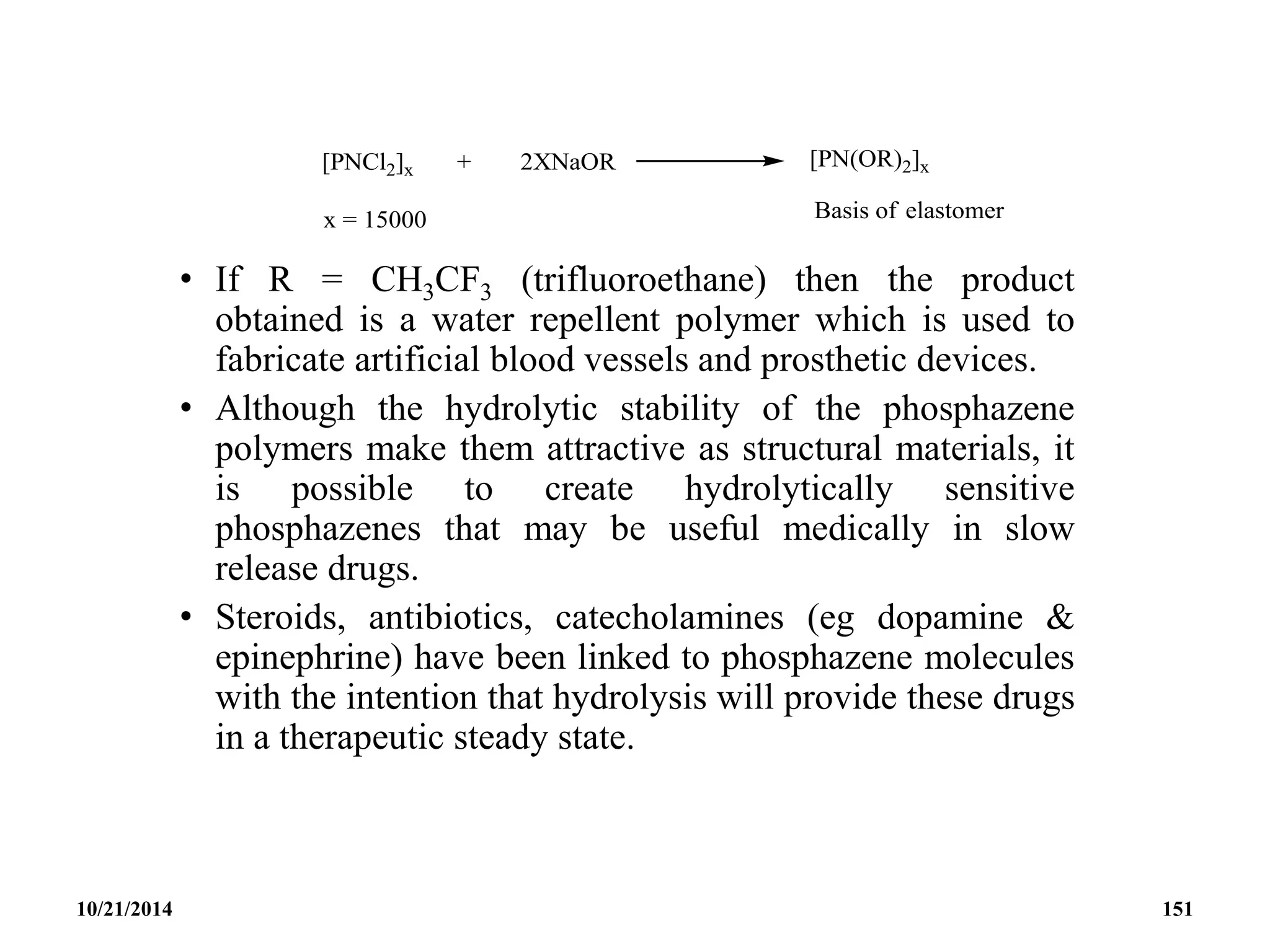
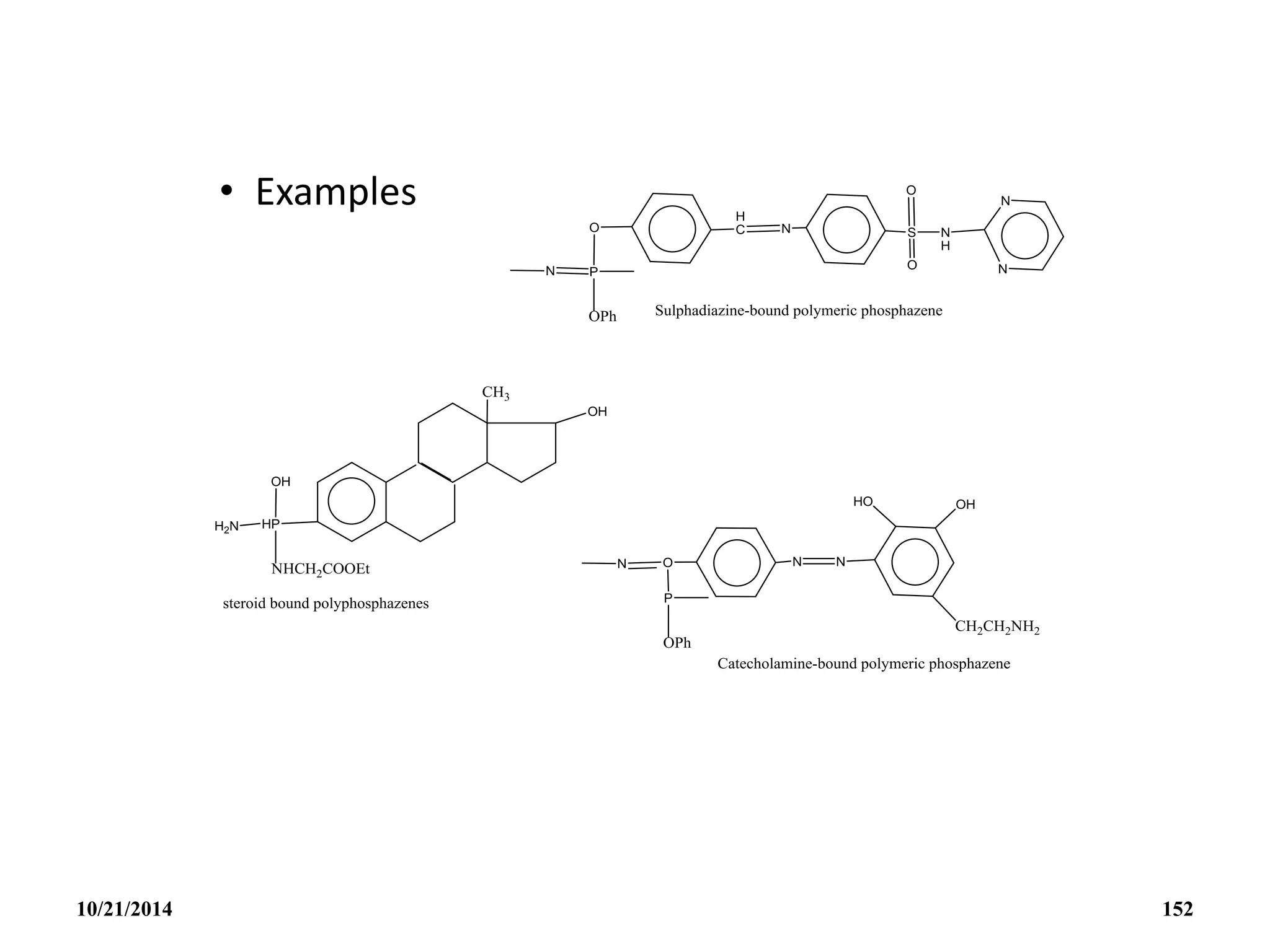
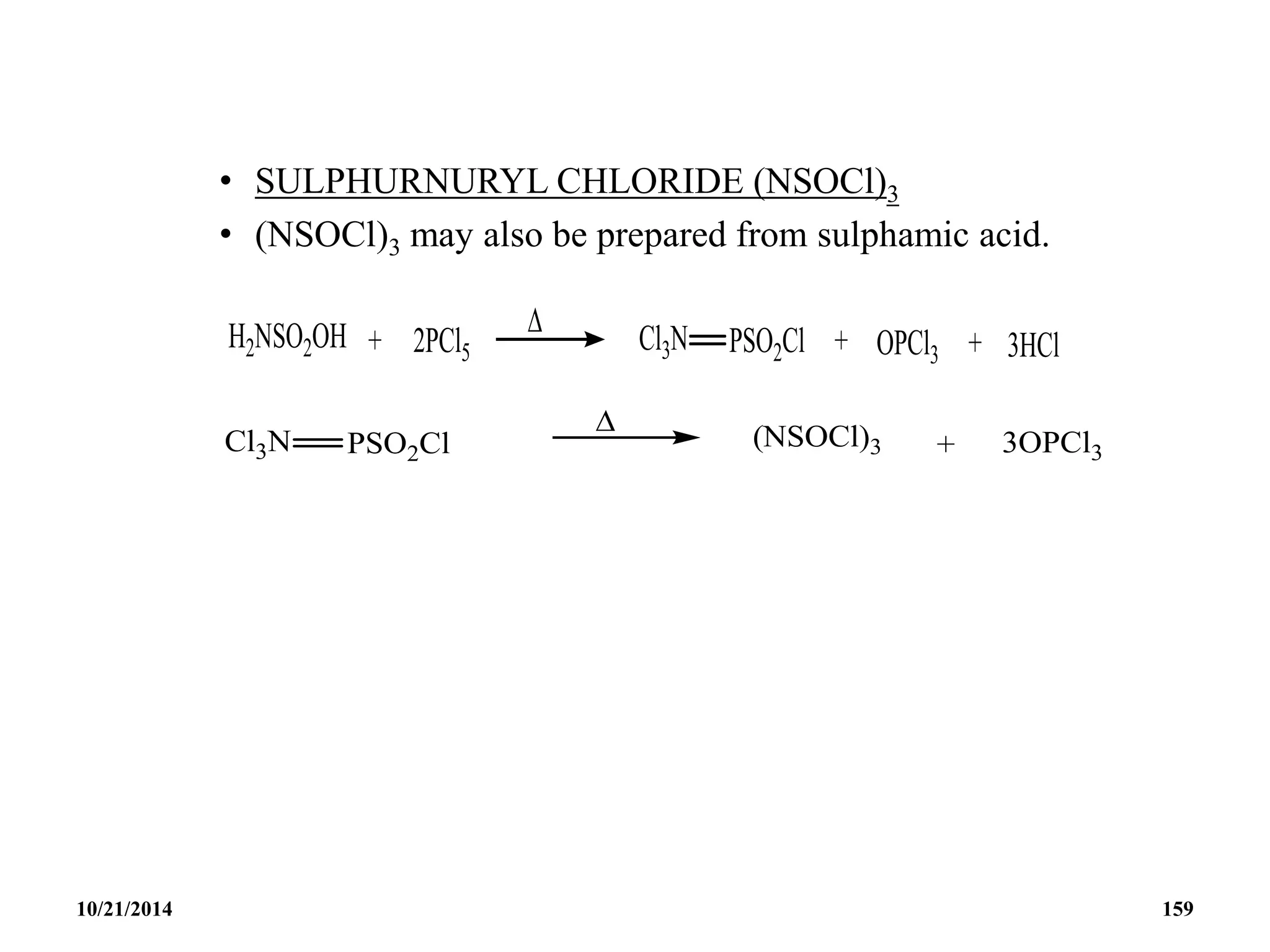
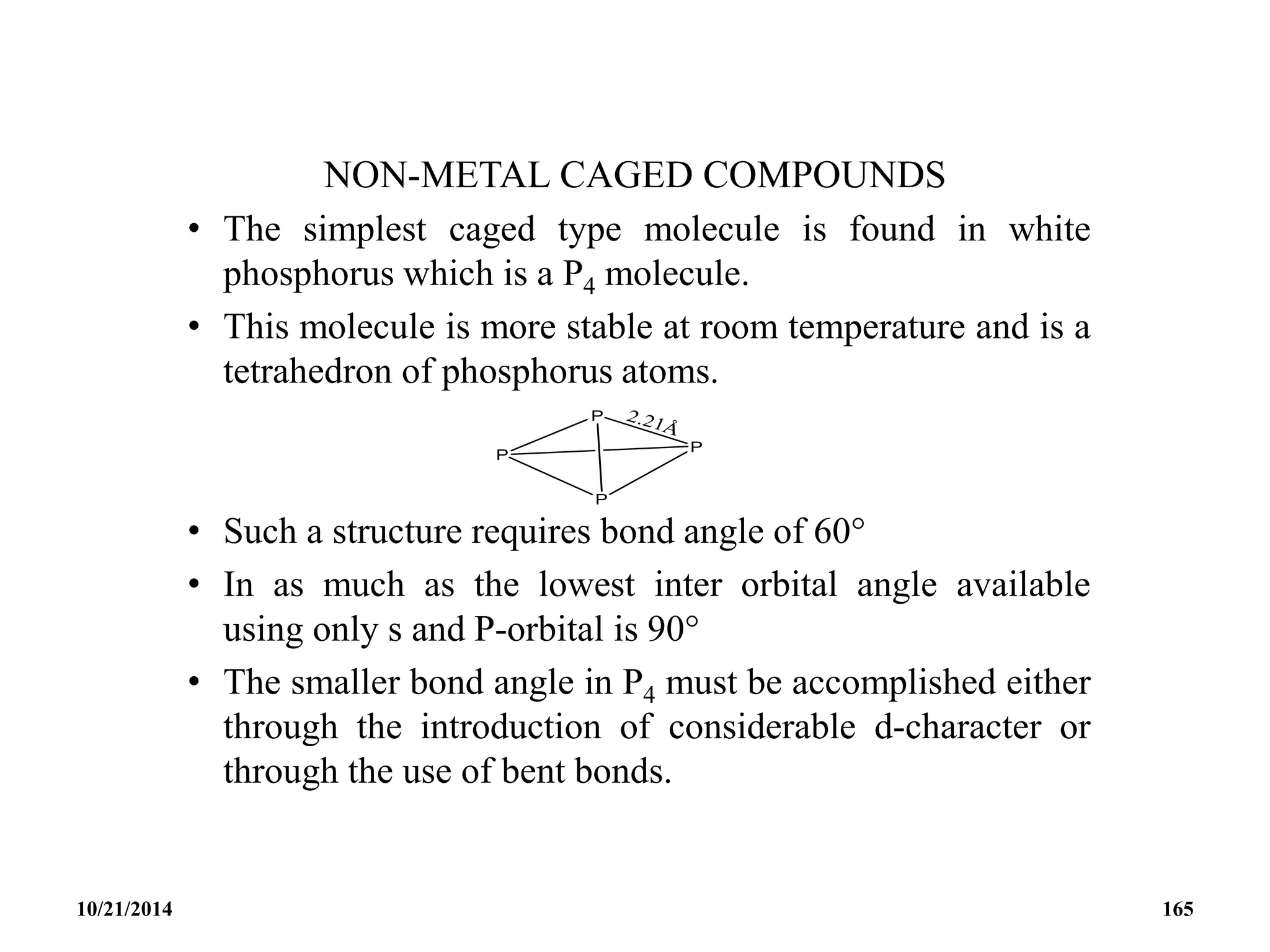
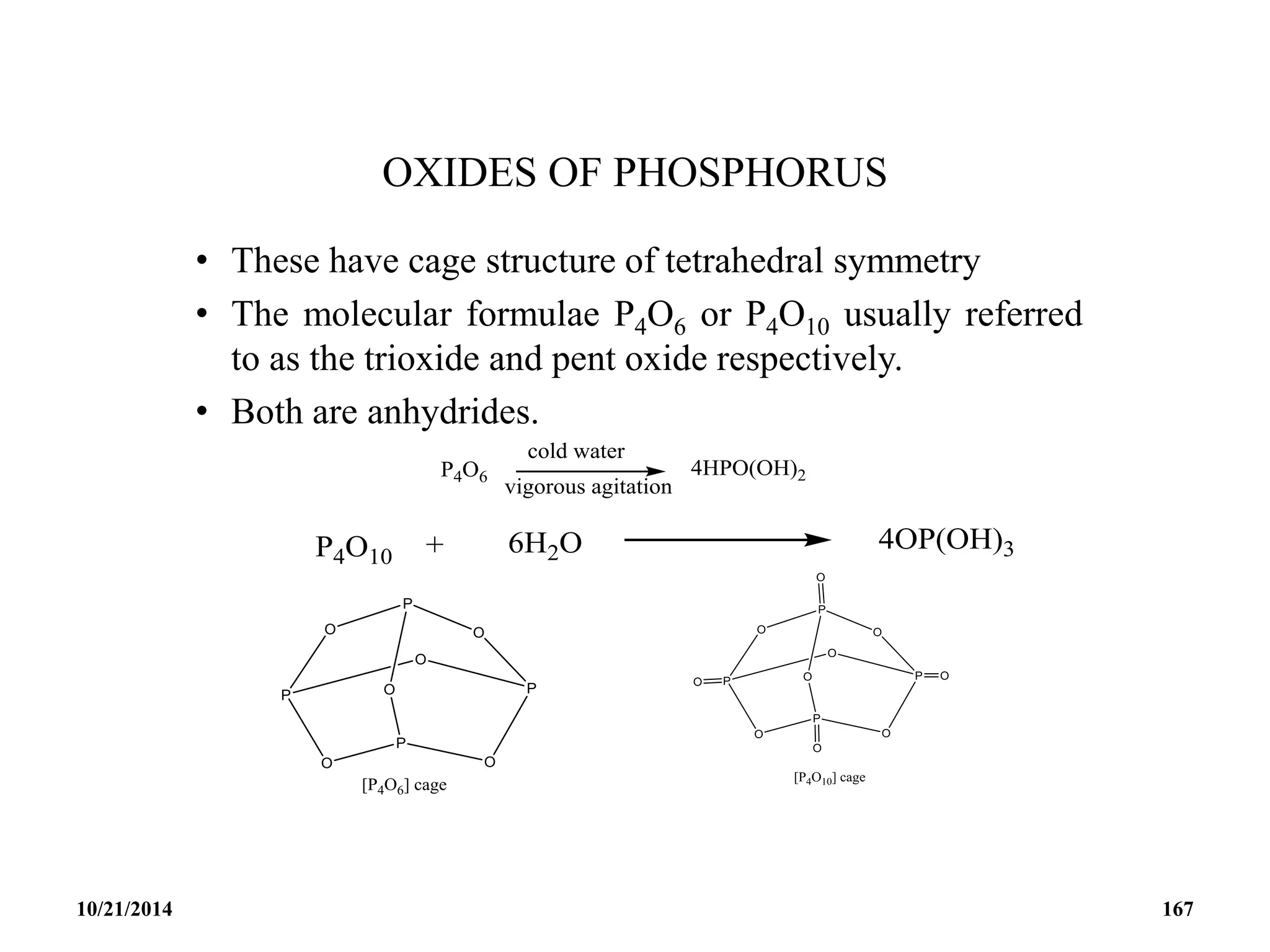
![PHOSPHAZENES (Phosphonitrilic compounds)
• These are cyclic compounds of phosphorus and nitrogen
of general formula [PNCl2]n.
• The reaction produces a mixture of ring compounds
(NPCl2)n, where n = 3,4,5,…. and fairly short linear
chains.
• The most common rings (n=3 and 4) contain six or eight
atoms.
• The former are flat and the latter exists in ‘ chair and boat
conformations.
10/21/2014 142](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-142-2048.jpg)
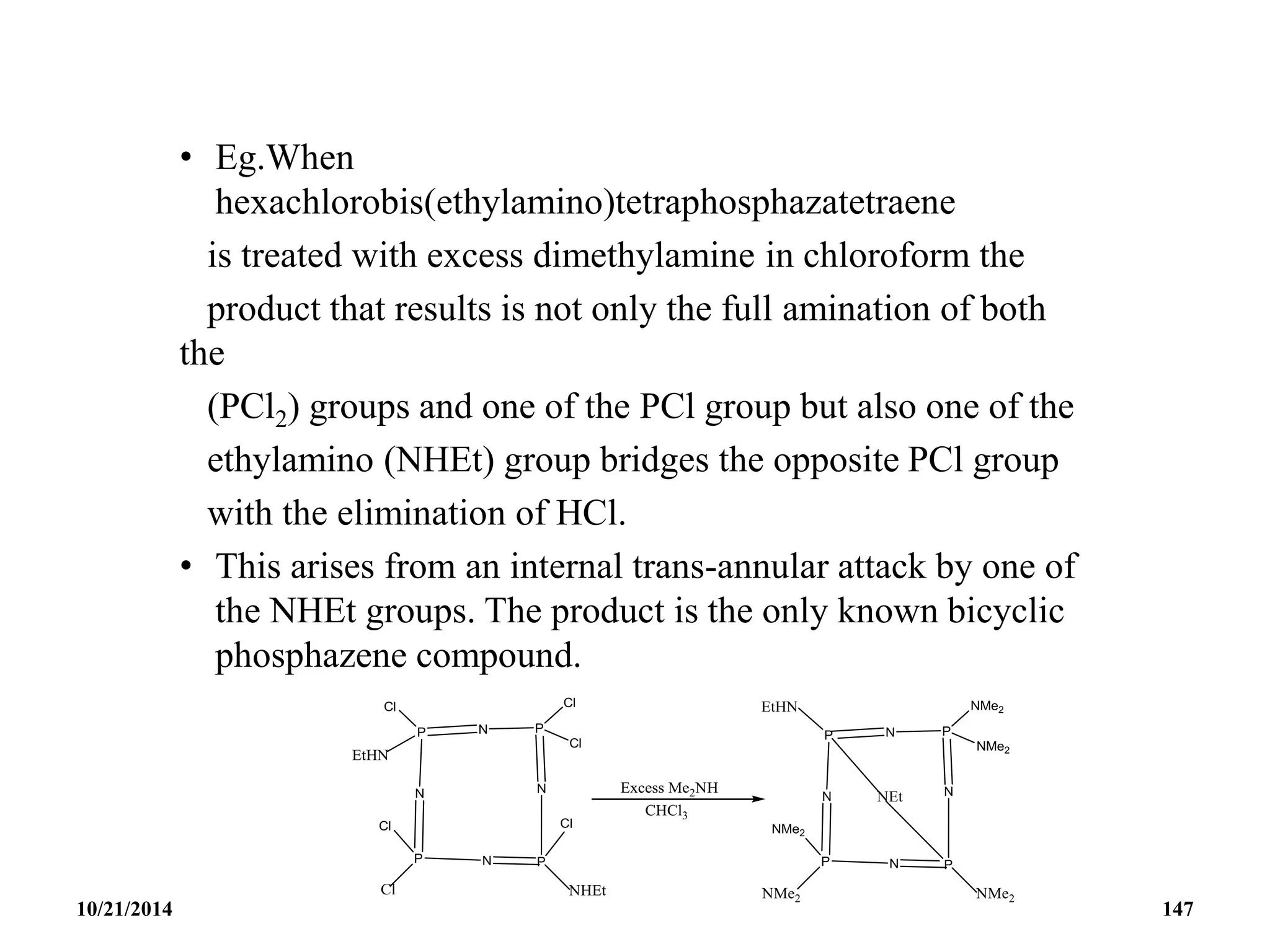
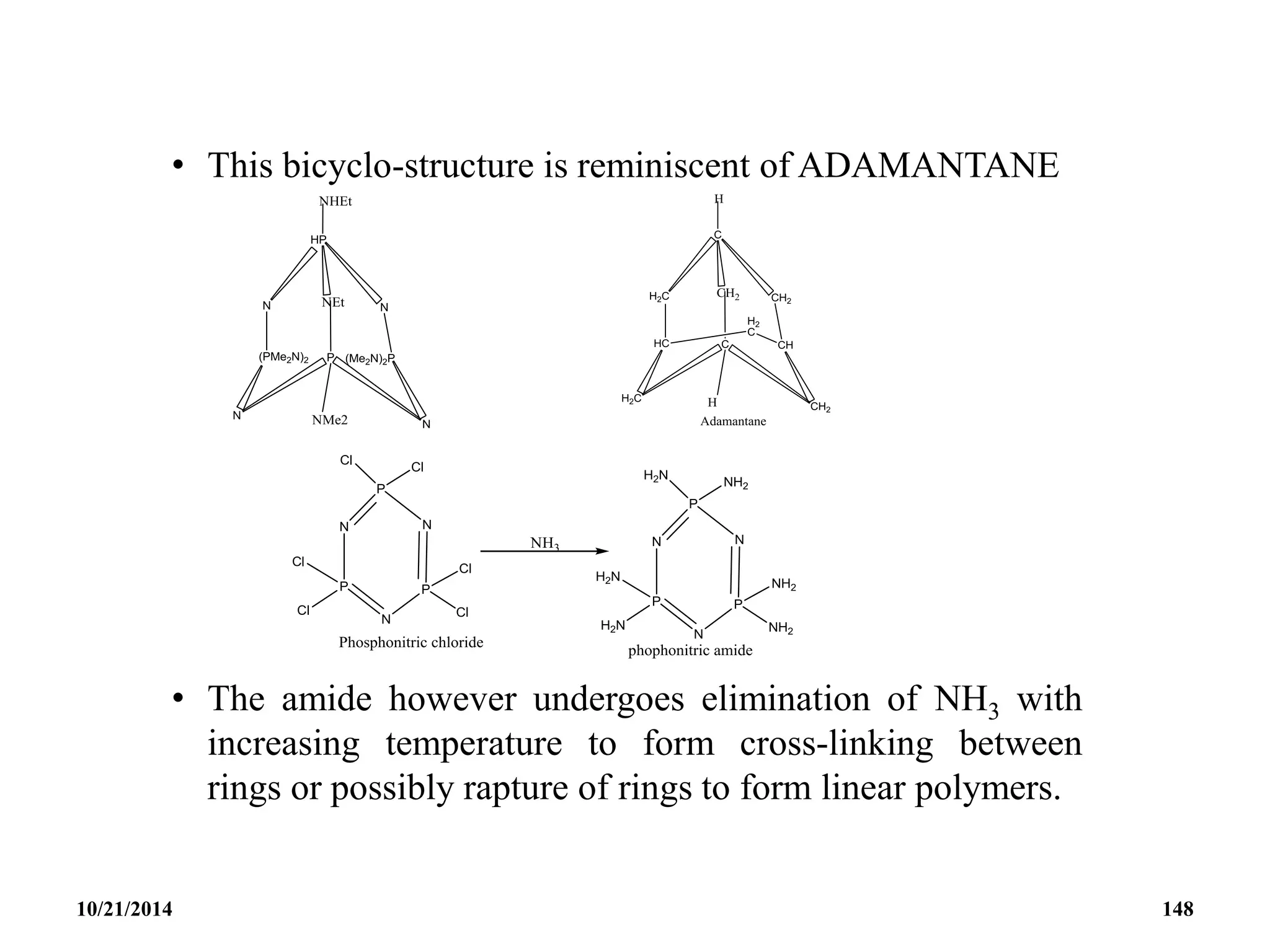
![Tetrameric Phosphonitriles or cyclotetraphosphaza-
tetraenes
• These are [NPCl2]4 and [NP(NMe2)2]4
• The tetrameric rings of the above compounds are more
flexible than those of the trimers.
• There is enough strain and they exhibit non-planar
structures without serious deterioration of the P─N –
bonding.
• The chloro-derivatives are useful for introducing other
substituents at the P-atoms in the ring.
10/21/2014 146](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-146-2048.jpg)
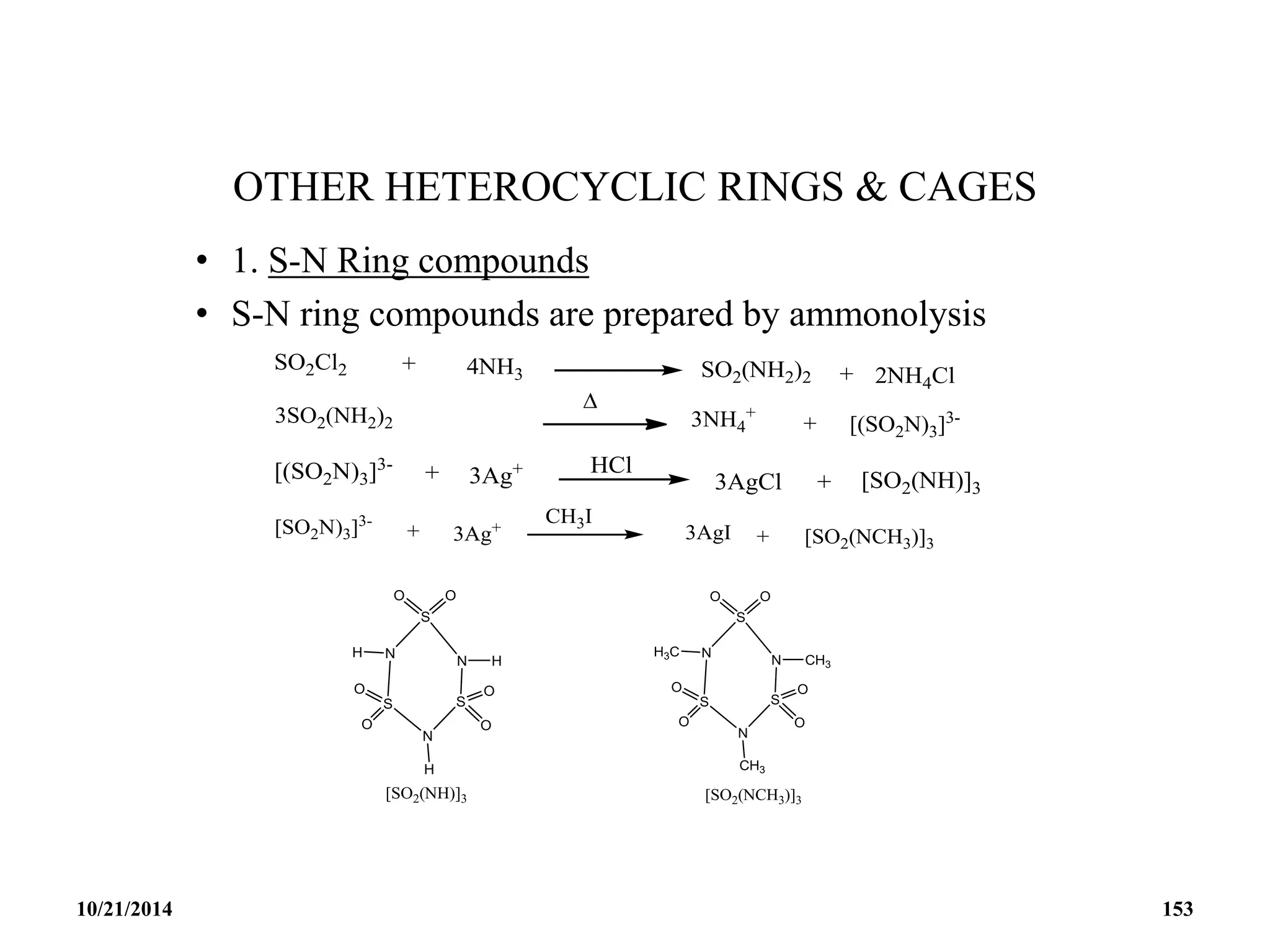

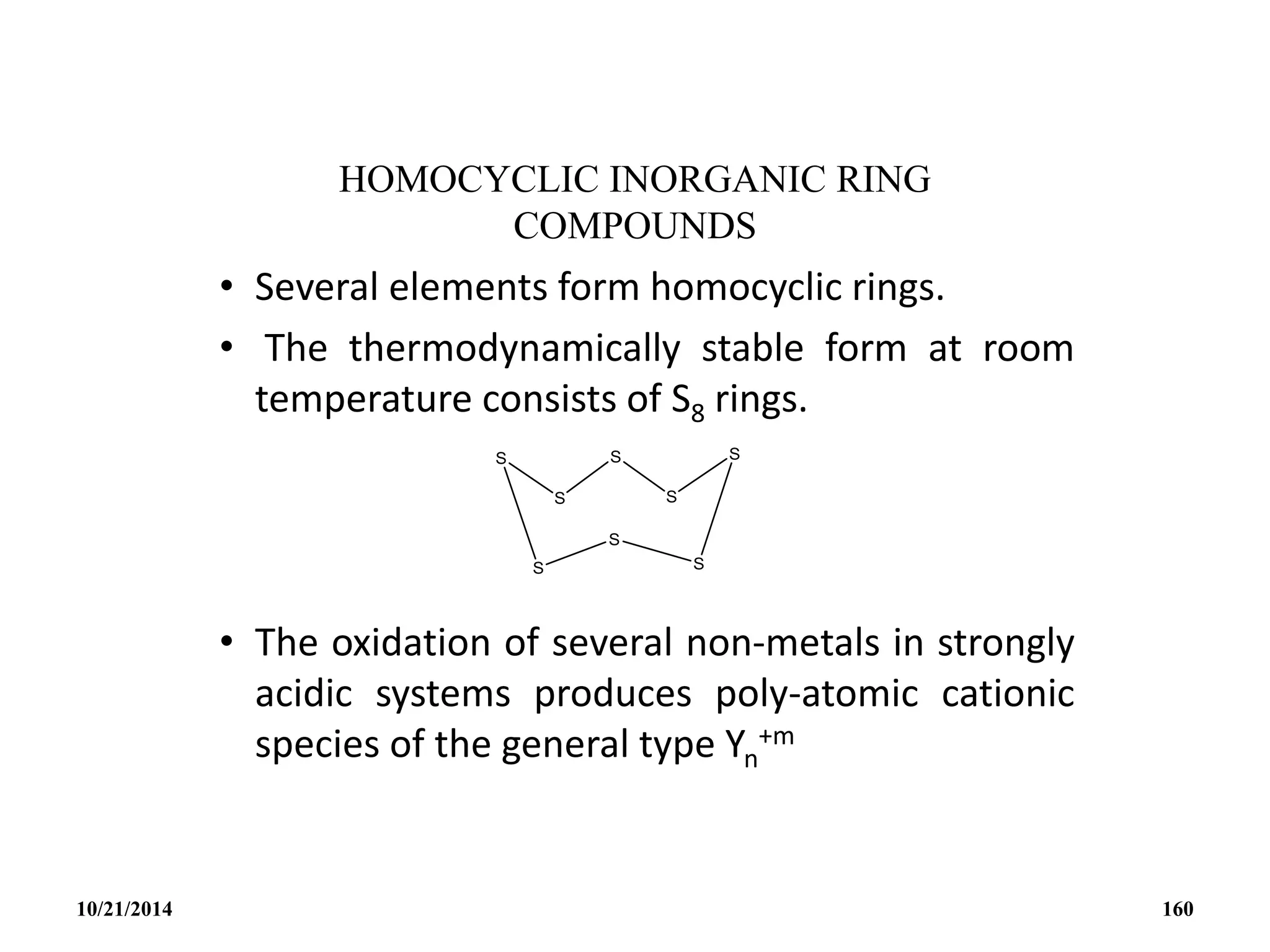
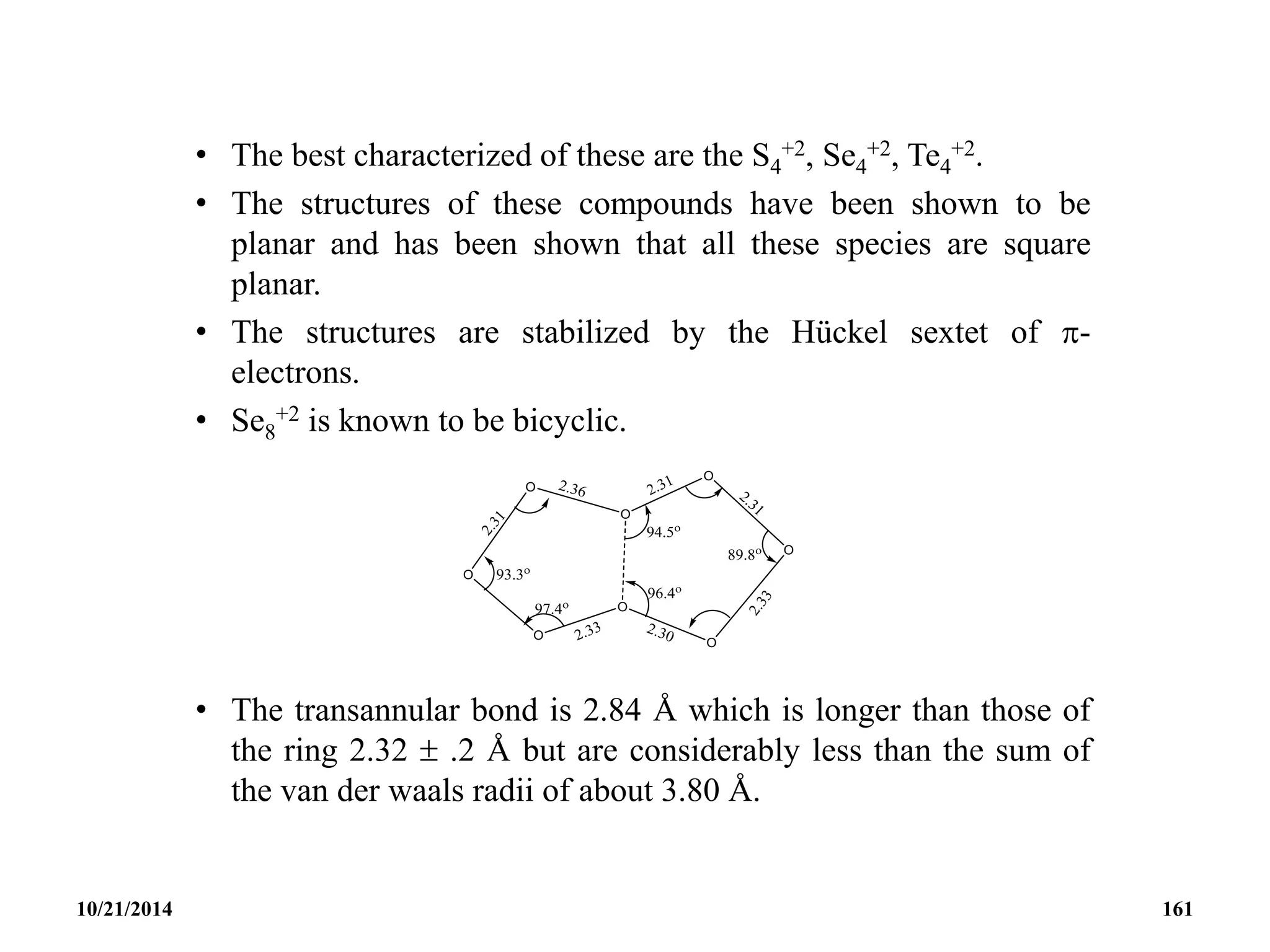
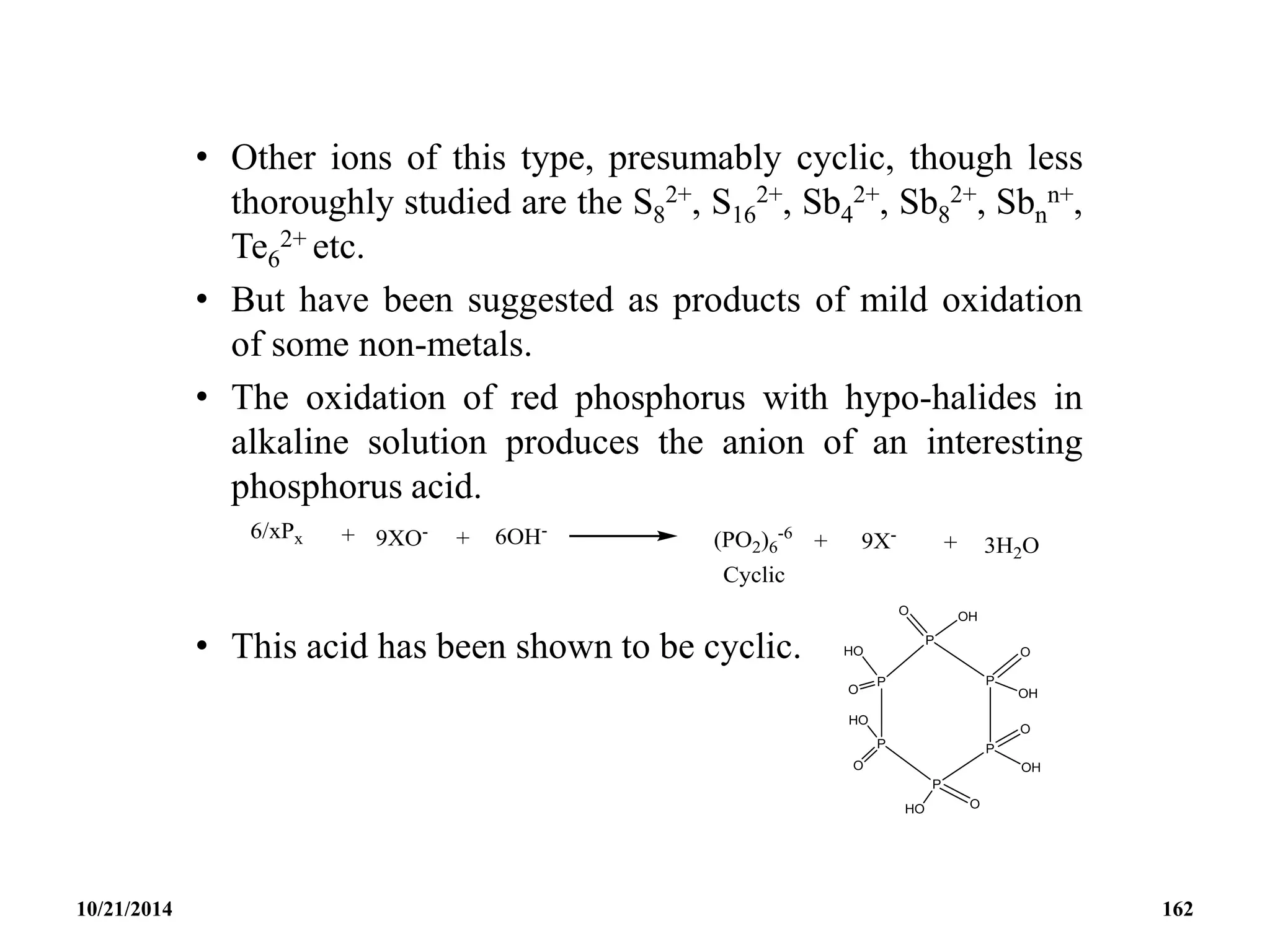
![• CYCLIC OXOCARBON ANION [(CO)n]-2 (-4)
• The oxocarbonate ion C5O5
2- is the first member to be
synthesized.
• It was isolated in 1825 by Gmelin and thus shares with benzene
the honour of being the first aromatic compound discovered.
• It was the first inorganic substance discovered that is aromatic.
• It is a bacterial metabolic product and was possibly the first
organic compound synthesized.
10/21/2014 163](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-163-2048.jpg)

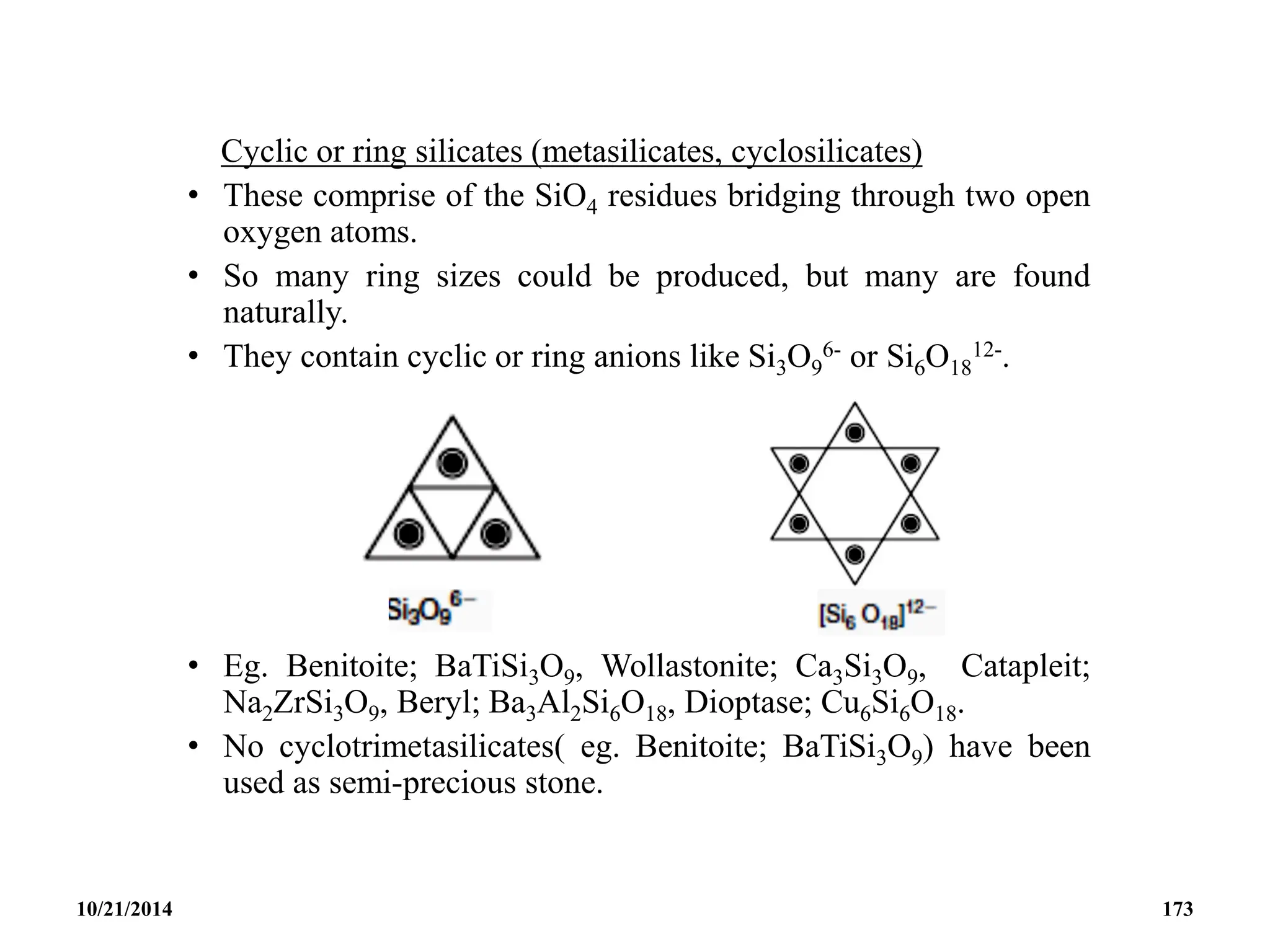
![• The cyclohexametasilicate ion Si6O18
12- occurs as beryl;
Ba3Al2Si6O18 in emeralds.
• Tourmarine is an example of a mixed borosilicate containing
this BO3
3- anions which occurs in basic salts eg.
[Al(OH)4(BO3)3Si6O18 ]7-.
• This ion occur in sapphire (blue) and topaz (pale yellow).
• The crystal structures show that the rings of Si6O18
12-occupy
sheets which are bound to others by metal ions in between
them.
Chain silicates
Pyroxene and Amphibole (Inosilicates)
• They contain the anions which are formed by sharing of two
oxygen atoms by each tetrahedra.
• The anions may be of the types (a) (SiO3)n
2n- -pyroxes, (b)
(Si4O11)n
6n- - amphibole
10/21/2014 174](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-174-2048.jpg)
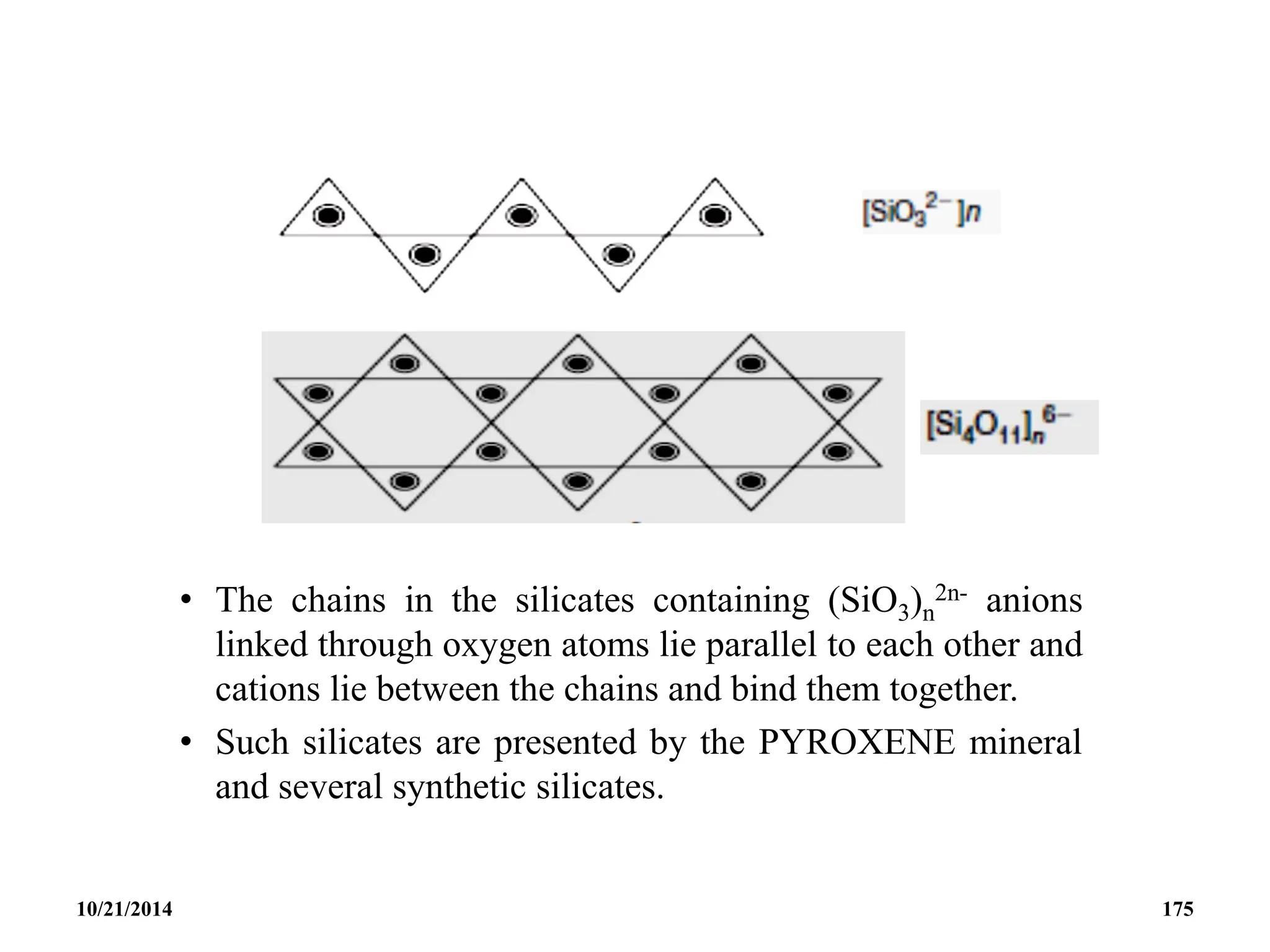
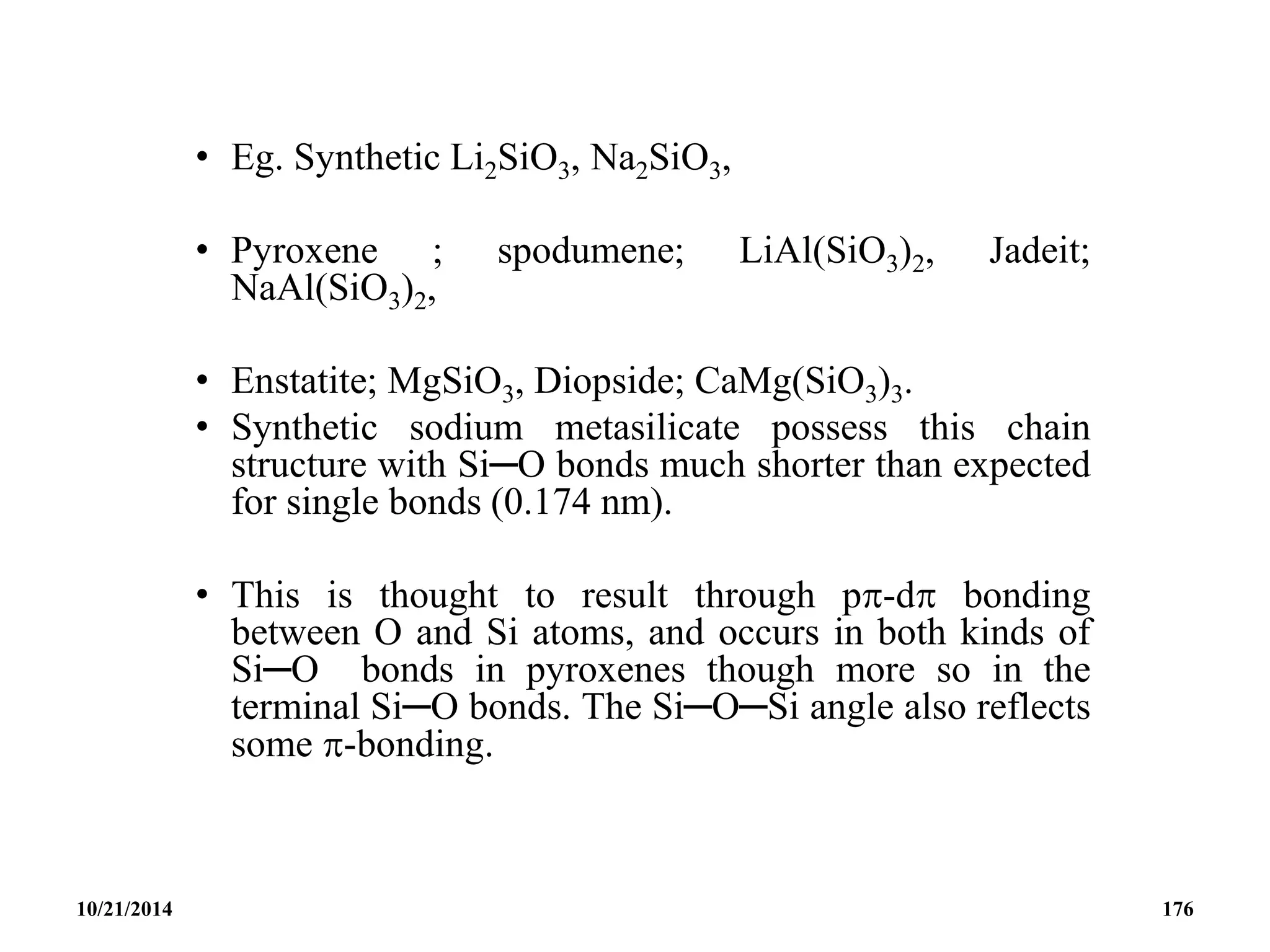
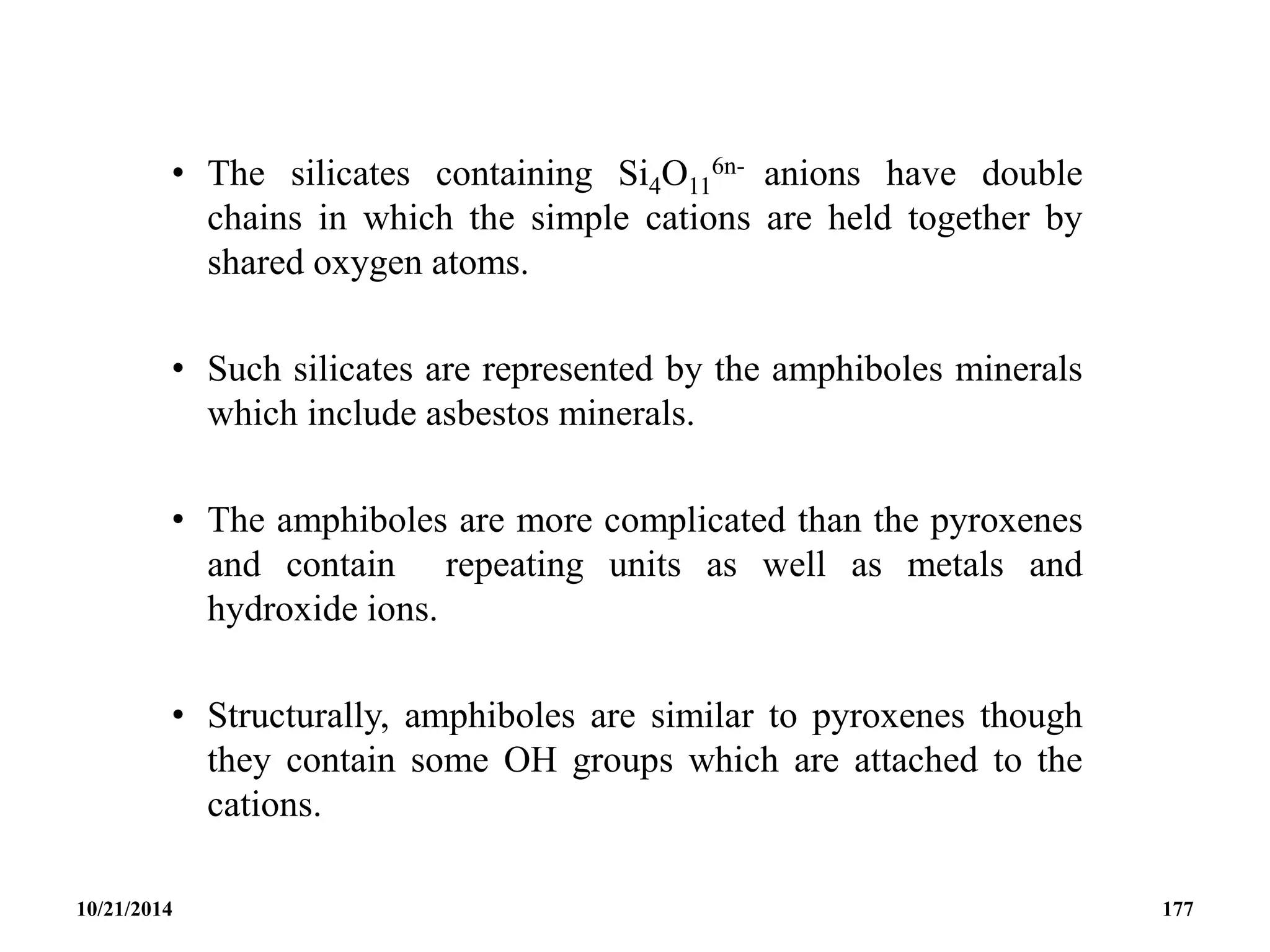
![• Eg. Tremolite; Ca2Mg5[(OH)2(Si4O11)2], Crocodolite;
Na2Fe3
2+Fe2
3+[(Si4O11)2]2(OH)2
Asbestos
• Asbestos was the term originally used to describe fibrous
amphiboles, but it now incorporates many two
dimensional polymers encountered among the
aluminosilicates.
• Thus chrysotile, once regarded as an amphibole
(OH)6MgSi4O11.H2O, is actually (OH)4Mg3Si2O5.H2O, the
Si2O5 anion having a layer structure.
• Replacing the Mg by Al gives the aluminosilicate
(OH)4Al2Si2O5 encountered in kaolin.
10/21/2014 178](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-178-2048.jpg)
![ZEOLITES (porotectosilicates)
• These are aluminosilicates with framework structures
enclosing cavities occupied by large ions and water molecules,
both of which have considerable free movements, permitting
ion-exchange, reversible hydration and absorption of gases.
• The framework consists of an open arrangement of corner-sharing
tetrahdra where SiO4 units are partially replaced by AlO4 tetrahedra
which require sufficient cations to achieve electroneutrality.
• The cavities are occupied by H2O molecules and the idealized
formula is Mx/n
n+[(AlO2)x(SiO2)y]x-.zH2O.
• Eg: Natrolite; Na2(Al2Si2O8).H2O, Heulandite;
Ca(Al2Si7O18).6H2O, Chabazite; Ca(Al2Si4O12).6H2O.
10/21/2014 184](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-184-2048.jpg)
![• The cation produces a wide range of colour and are used
for pigments.
• E.g. Ultramarine; Na8[(AlSiO4)]S2, Sodalite;
Na8[(AlSiO4)6]Cl2, Nosean; Na8[(AlSiO4)6]SO4.
The Si4─Si6 ring layer structure
• This involves alternate Si4O4 and Si8O8 rings but is rare.
• Apophihyllite (K,Na)Ca4Si8O20(F,OH).8H2O has such a
structure with terminal oxygen atoms of one Si4O4 ring
directed to the opposite side of the layer to its Si4O4
neighbour.
• Cations holds the layers together.
10/21/2014 186](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-186-2048.jpg)

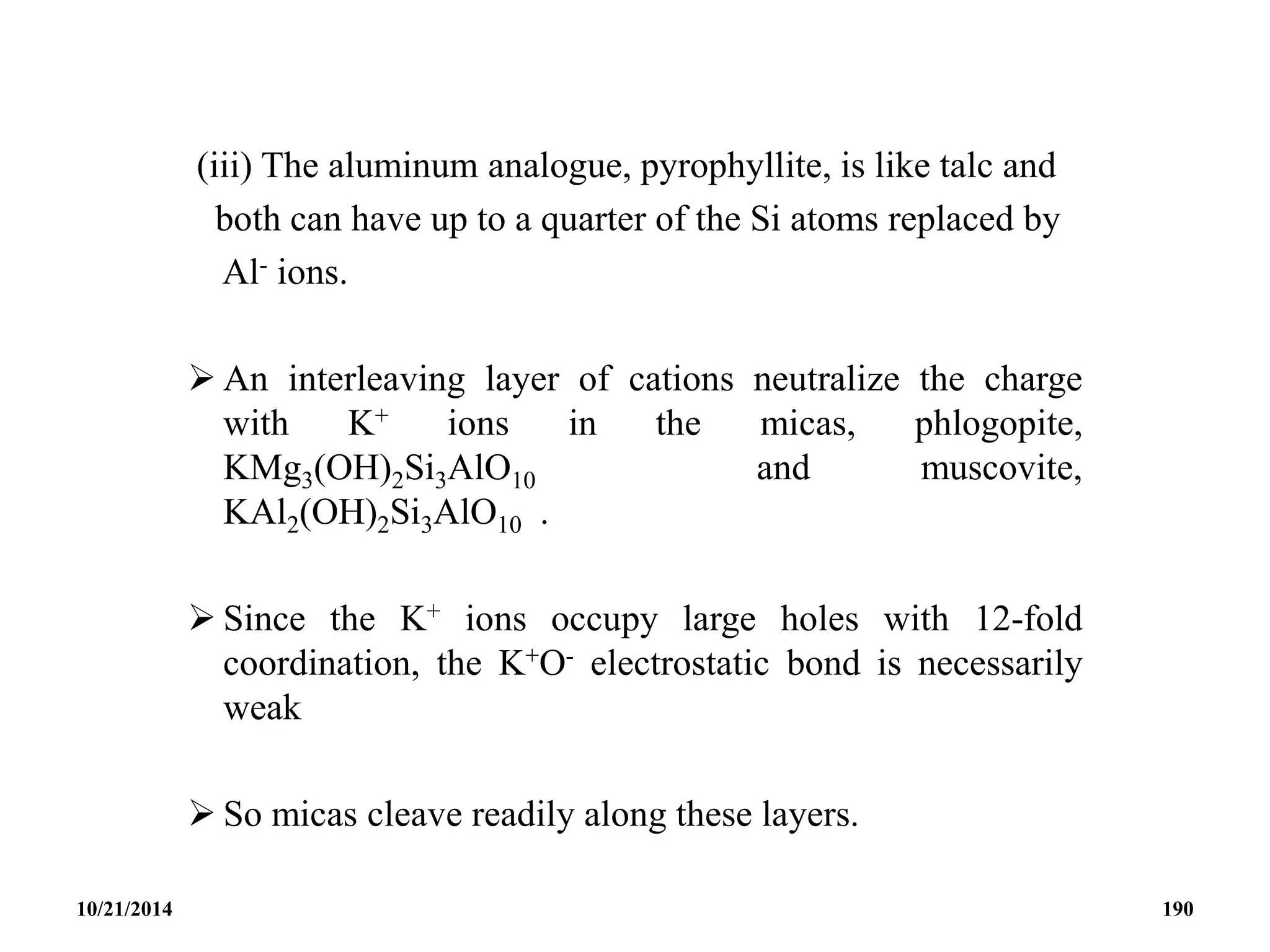

![ Further replacement of both cation and Si atoms in talc, and
interleaving with hydrated Mg2+ ions produces vermiculite,
(Mg,Fe,Al)3(Al,Si)4O10(OH)2.4H2O -
[Mg2.36FeIII
0.48Al0.16)(Si2.72Al1.28)O10(OH)2]-0.64[Mg0.32(H2O)4.32]+0.64.
This dehydrates readily to a talc-like structure, and is used
widely as a soil conditioner and porous filler.
Relative Hardness of Typical Hydroxysilicates
Hydroxysilicate Formula Hardness (Moh’s scale)
Talc Mg3(OH)2Si4O10 1 - 2
Mica KMg3(OH)2Si3AlO10 2 -3
Brittle CaMg3(OH)2Si2Al2O10 31/2 - 5
10/21/2014 192](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-192-2048.jpg)
![ Monimorillonite, [Mg1/3Al12/3Si4AlO10(OH)2]-1/3 results
from pyrophyllite by replacing one sixth of the Al3+ ions
by Mg2+ ions.
Like many clays minerals, this can be readily hydrated and
exhibits cation–exchange properties.
It is an important constituent of Fullers earth, found
widely in Southern England.
(v) With kaolin, talc and pyrophyllite the layers are
uncharged and so only weakly bound together, while the
micas are held by cation layers or hydrated cations (eg
vermiculite).
Interleaving with charged hydroxide layers gives the
chlorite minerals.
10/21/2014 193](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-193-2048.jpg)
![ Thus the mica layers (composition [Mg3(AlSi3O10) (OH)2]- to
[Mg2Al(Al2Si2O10) (OH)2]- are held by ions.
Thus in phlogopite, KMg3(OH)2Si3AlO10), replacing the K+ ion
by the Mg2Al(OH)6
+ ion gives a chlorite mineral.
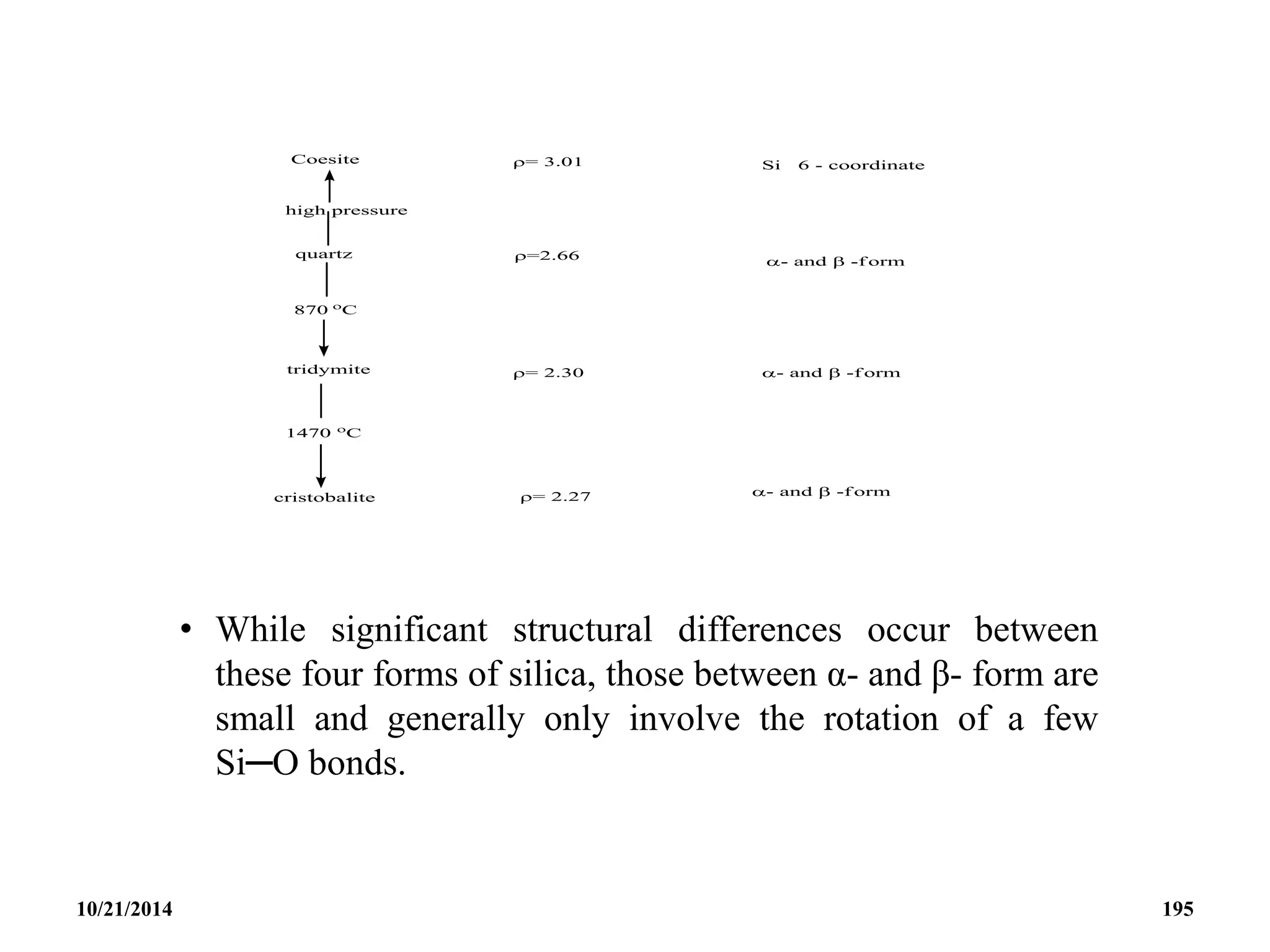
Silica, SiO2
• This has a structure comprising of silicon atoms
tetrahedrally surrounded by four oxygen atoms.
• Polymerization occurs in a variety of ways, and
transmission between the various crystalline forms occur
but with much difficulty.
• The high temperature form have the more open structures,
while the high pressure ones are more compact as would
be expected from the Le Chatelier’s principle.
10/21/2014 194](https://image.slidesharecdn.com/chem351inorganicpolymersandelectrondeficientcompounds2-240208114404-4cc4e446/75/CHEM-351-INORGANIC-POLYMERS-AND-ELECTRON-DEFICIENT-COMPOUNDS-2-pdf-194-2048.jpg)